1
DESCRIPTION
Natrelle
®
breast implants and tissue expanders are designed for use in
augmentation and reconstruction mammoplasty. All Natrelle
®
implants and
expanders are constructed of a silicone elastomer shell and are latex free.
- Natr elle
®
single lumen gel-filled breast implants are pre-filled with cohesive
silicone gel designed to simulate natural breast tissue.
- Natrelle
®
saline-filled breast implants are filled with saline at the time of surgery.
- Natrelle
®
133 tissue expanders are intended for temporary subcutaneous
implantation to develop surgical flaps and additional tissue coverage.
- Natrelle
®
150 double lumen gel/saline breast implants are designed to function
as both tissue expanders and long-term breast implants for one-stage breast
reconstruction or augmentation.
- Natrelle
®
breast implants and tissue expanders contain no latex or natural rubber
materials.
IMPLANT DESIGN FEATURES
Gel and Gel/Saline Implants
• All Natrelle
®
textured gel and gel/saline implants have a BIOCELL™ textured
surface engineered with a deep open pore design for firm tissue adherence.
• The INTRASHIEL™ shell features a patented barrier coat between two layers of
silicone elastomer to minimise gel diffusion.
• The Natr elle
®
150 includes a magnetic resonance imaging (MRI) compatible
self-sealing Mini Remote Injection Site which contains a titanium needle guard
to prevent inadvertent puncture through the base of the injection site.
• Anatomically shaped Natrelle
®
single lumen gel-filled breast implants include
orientation dots to aid in correct implant positioning during surgery (see
Figure 1).
Saline Implants
• Diaphragm valves in Natrelle
®
saline-filled breast implants are designed for ease
in filling and subsequent air removal.
Tissue Expanders
• BIOCELL™ textured surface is designed to promote tissue adherence.
• The stable base in the Natrelle
®
133 provides greater control over expansion
direction.
• Natrelle
®
133 tissue expanders, with integral MAGNA-SITE™ injection site are
supplied with a MAGNA-FINDER™ external locating device. The MAGNA-
SITE™ and MAGNA-FINDER™ contain rare-earth, permanent magnets for an
accurate injection site locating system. In vitro tests show that the MAGNA-
SITE™ is detectable through 60mm of phantom tissue.
• All injection sites are self-sealing and contain a titanium needle guard to prevent
inadvertent puncture through the base of the injection site (see Figure 2).
NATRELLE
®
ACCESSORIES
• Fill Tube Plug Kits;
• MAGNA-FINDER™
• Other product accessories are available separately.
EACH PATIENT MUST BE INDIVIDUALLY EVALUATED FOR IMPLANT
SURGERY BASED ON THE CLINICAL JUDGEMENT OF A QUALIFIED
SURGEON.
INDICATIONS
• Unilateral or bilateral hypoplasia of the breast.
• Breast reconstruction in patients with adequate tissue covering following
mastectomy or trauma.
• Asymmetry, ptosis, or aplasia of the breast.
• Replacement of implants for medical or cosmetic reasons.
• Congenital deformity of the breast.
• Breast reconstruction in patients following mastectomy or trauma in the case of
tissue expanders.
• Treatment of soft tissue deformities in the case of tissue expanders.
• A patient deemed suitable for breast augmentation must be at least 18 years old
(22 years old in Singapore).
CONTRAINDICATIONS
• Tissue covering determined inadequate or unsuitable by the surgeon.
• Active infection, local and systemic.
• Existing carcinoma of the breast without mastectomy and residual gross local
tumour of the breast after mastectomy.
• Advanced fibrocystic disease considered to be pre-malignant without
mastectomy.
• Use of drugs that might result in high surgical risk and/or significant postoperative
complications, including drugs that would interfere with blood clotting.
• A patient that demonstrates or shows signs of psychological instability (i.e., an
inappropriate attitude or motivation).
• Women who are currently pregnant or breastfeeding.
• Natrelle
®
133 tissue expanders contain a MAGNA-SITE™ and should not be
used in patients who already have implanted devices that would be affected by a
magnetic field (e.g., pacemakers, drug infusion devices).
• Diagnostic testing with MRI is contraindicated in patients with Natrelle
®
133
tissue expanders in place. The MRI equipment could cause movement of the
MAGNA-SITE™ tissue expander and result in not only patient discomfort
but also tissue expander displacement, requiring revision surgery. Also,
the MAGNA-SITE™ magnet can interfere with MRI and X-ray detection
capabilities. All other Natrelle
®
implants are MRI safe.
FIGURE 1
Location of Orientation Dots
Indicates dot location on all sizes.
Indicates additional dot location on selected styles and sizes.
Anterior View
Posterior View
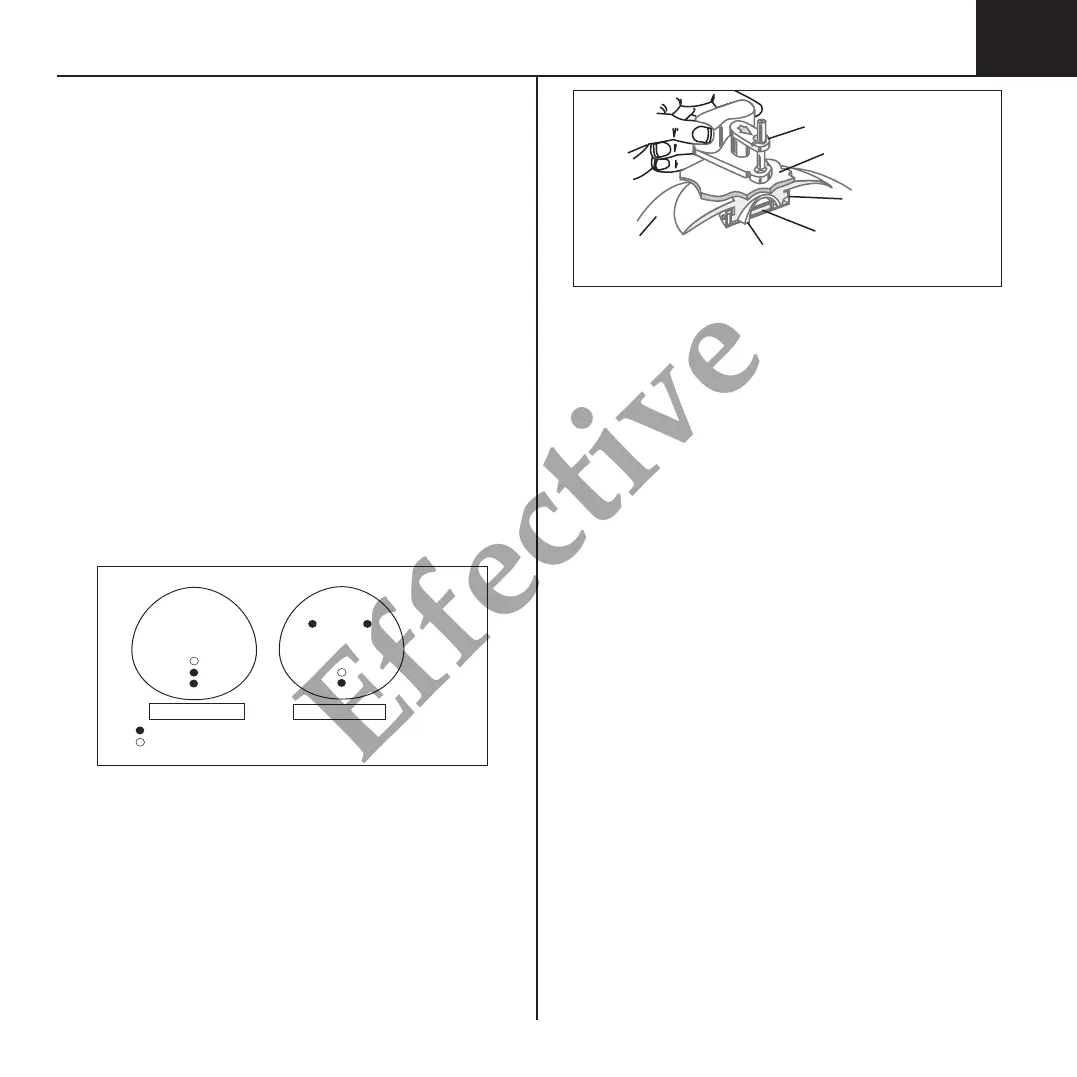
FIGURE 2
MAGNA-SITE™ & MAGNA-FINDER™ Locating System
Silicone
Expansion
Envelope
Self-Sealing
Silicone
Membrane
MAGNA-FINDER™
Overlying
Expanded Tissue
Puncture-Proof
Titanium Needle Guard
Rare-Earth
Permanent Magnet
NATRELLE® B I T E
E
N
Release Date: 29 Sep 2015 00:07:57 GMT -07:00
Expires one day from 21 Dec 2015
Effective
 Loading...
Loading...