Instructions for use
Amazon COVID-19
Test Collection Kit
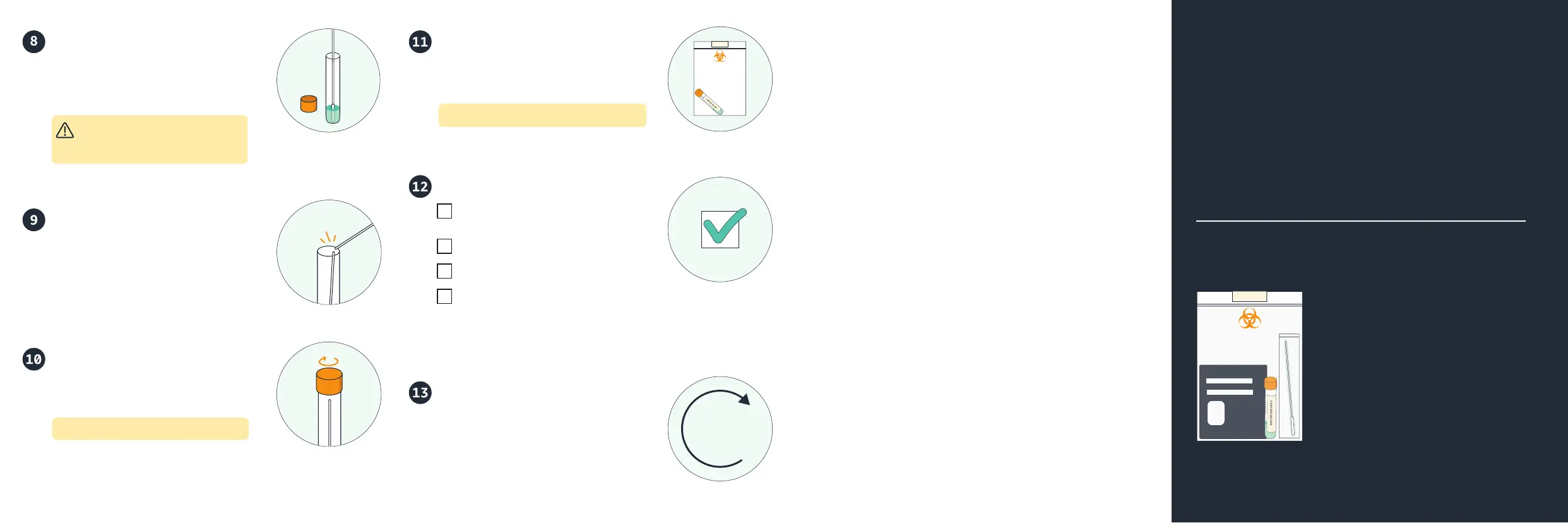
Put swab in collection tube
While holding the swab, open the tube.
Put the tube cap with the flat top down
on a clean surface nearby. Put the swab
soft tip down in the tube.
Do not spill the fluid in the tube!
If you spill the fluid, clean the area
and discard your kit components.
Break off the swab handle
To break off the swab handle, hold
the tube firmly. Bend the swab
handle at the indicator line back and
forth against the collection tube until
it breaks. Discard the swab handle.
Recap the tube
Make sure the cap is on tight, so that
no fluid can spill out. If the tube leaks
we cannot process your sample.
Wash your hands (or use hand sanitizer).
Put tube inside the plastic bag
Put the sealed tube inside the plastic
bag. Release excess air from the bag
and seal it shut.
Clean the surface area with a disinfectant.
Checklist before returning your bag
I registered my kit (if not, go back to
steps 1-3).
I did not spill the fluid in the tube.
I collected a sample from both nostrils.
The nasal swab only touched my nostrils.
If you have not checked all of the boxes, your
test cannot be processed. Discard your kit
components and get a new kit.
Drop off your bag at your worksite
Within 24 hours drop off your sealed bag
at your designated worksite. Once your
sample has been sent to the lab, you will
receive updates via e-mail/SMS.
24
hours
Questions?
covid19testing@amazon.com
E-mail
amazondx.com/testing-faq
Frequently asked questions
The emergency use of this product is only authorized for the duration of the
declaration that circumstances exist justifying the authorization of emergency
use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under
Section 564(b)(1) of the Federal Food, Drug and Cosmetics Act, 21 U.S.C. §
360bbb-3(b)(1), unless the declaration is terminated or authorization is
revoked sooner.
410 Terry Ave N, Seattle, WA 98109
For In Vitro Diagnostic Use Only. For Emergency Use Authorization Only.
Rx Only. For Use by Individuals 18 Years or Older.
This product has not been FDA cleared or approved but has been authorized
for emergency use by FDA under an EUA for use by authorized laboratories.
This product has been authorized only for the detection of nucleic acid from
SARS-CoV-2, not for any other viruses or pathogens.

 Loading...
Loading...