Do you have a question about the Bayer HealthCare MEDRAD Avanta and is the answer not in the manual?
| Brand | Bayer HealthCare |
|---|---|
| Model | MEDRAD Avanta |
| Category | Medical Equipment |
| Language | English |
Refer to warnings and cautions on instructions for use packaged in each carton.
Indicates device conforms to EU Medical Device Regulation 2017/745.
Authorized Representative in the European Community.
Identifies the manufacturer of the device.
Advises of circumstances that could result in injury or death to patient or operator.
Advises of circumstances that could result in damage to the device.
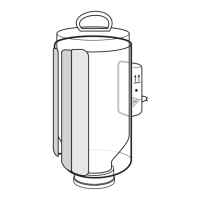
Identifies the footswitch connector.
For medical professionals with adequate training and experience in system operation and procedures.
Specifically for use with the MEDRAD Avanta Fluid Management Injection System.
Only intended for use with MEDRAD Avanta Fluid Management Injection System.
Potential patient or operator injury if package is opened/damaged, or damaged components are used.
Component damage may occur if not installed properly; ensure secure connections.
Steps for connecting the footswitch to the Fluid Control Module and system.
 Loading...
Loading...