Caution
Federal Law (U.S.A.) restricts this device to sale, distribution, or use by or on the order of a physician or properly licensed practitioner.
EXOGEN is only intended for use by the individual for whom it is prescribed. EXOGEN is for single patient use ONLY.
THIS DEVICE IS NON-STERILE.
It does not require sterilization before use.
EXOGEN is a Class B digital apparatus which meets all requirements of the Canadian Interference-Causing Equipment Regulations.
EXOGEN est un appareil de classe B qui répond a toutes les exigences des Régulations Canadiennes sur les équipements pouvant
causer des interférences.
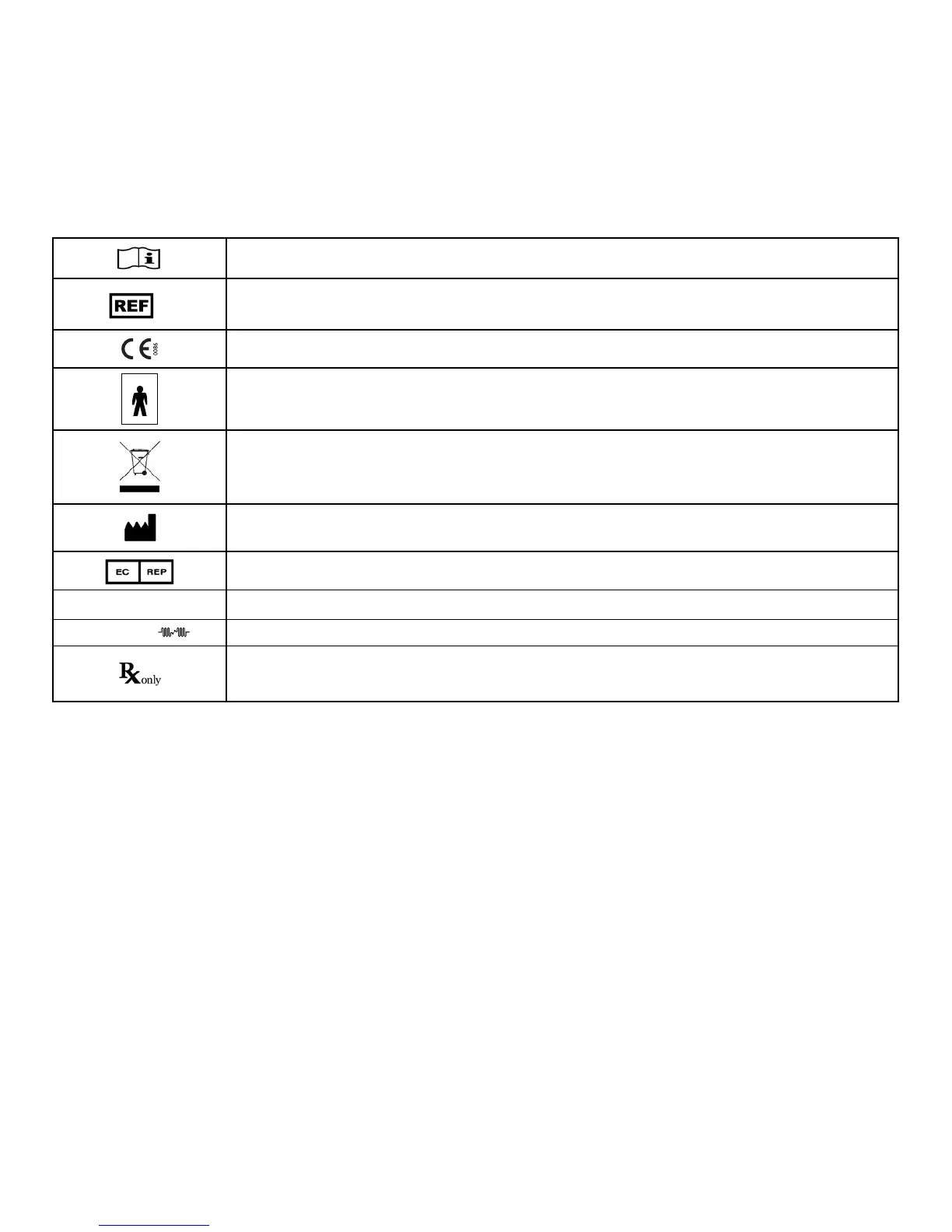
EXOGEN Label Symbol Descriptions and Equipment Classication
Information Symbol: refer to Instructions for Use.
Catalog Number
CE Mark: indicates conformity with European Council Directive of 14 June 1993 concerning Medical Devices
(93/42/EEC).
Type BF Applied Part. The transducer, shown in Figure 2 on page 2 is an applied part.
EU: Not for General Waste.
This symbol indicates that EXOGEN should not be disposed of with ordinary household waste at the end of its
life. For details on how to dispose of this device correctly, contact your local government waste disposal agency
or your local Bioventus representative.
Manufacturer
This symbol indicates the authorized representative in the European Community.
SN
Serial number (rst four digits of the serial number indicate the month and year of manufacture)
WAVEFORM
Pulsed Signal
Rx Symbol: Federal Law (U.S.A.) restricts this device to sale, distribution, or use by or on the order
of a physician or properly licensed practitioner. This device is only intended for use by the individual
for whom it is prescribed.
 Loading...
Loading...