ELITech Clinical Systems SAS
Zone Industrielle
61500 SEES
France
For Technical questions, Please call or contact
(855) 354-8324 - www.elitechgroup.com
27 Wellington Road
Lincoln, Rhode Island 02865 - U.S.A.
ELITech Clinical Systems SAS
Zone Industrielle
61500 SEES
France
For Technical questions, Please call or contact
(855) 354-8324 - www.elitechgroup.com
27 Wellington Road
Lincoln, Rhode Island 02865 - U.S.A.
(10/2015)
FTEVY-HDLL-v5
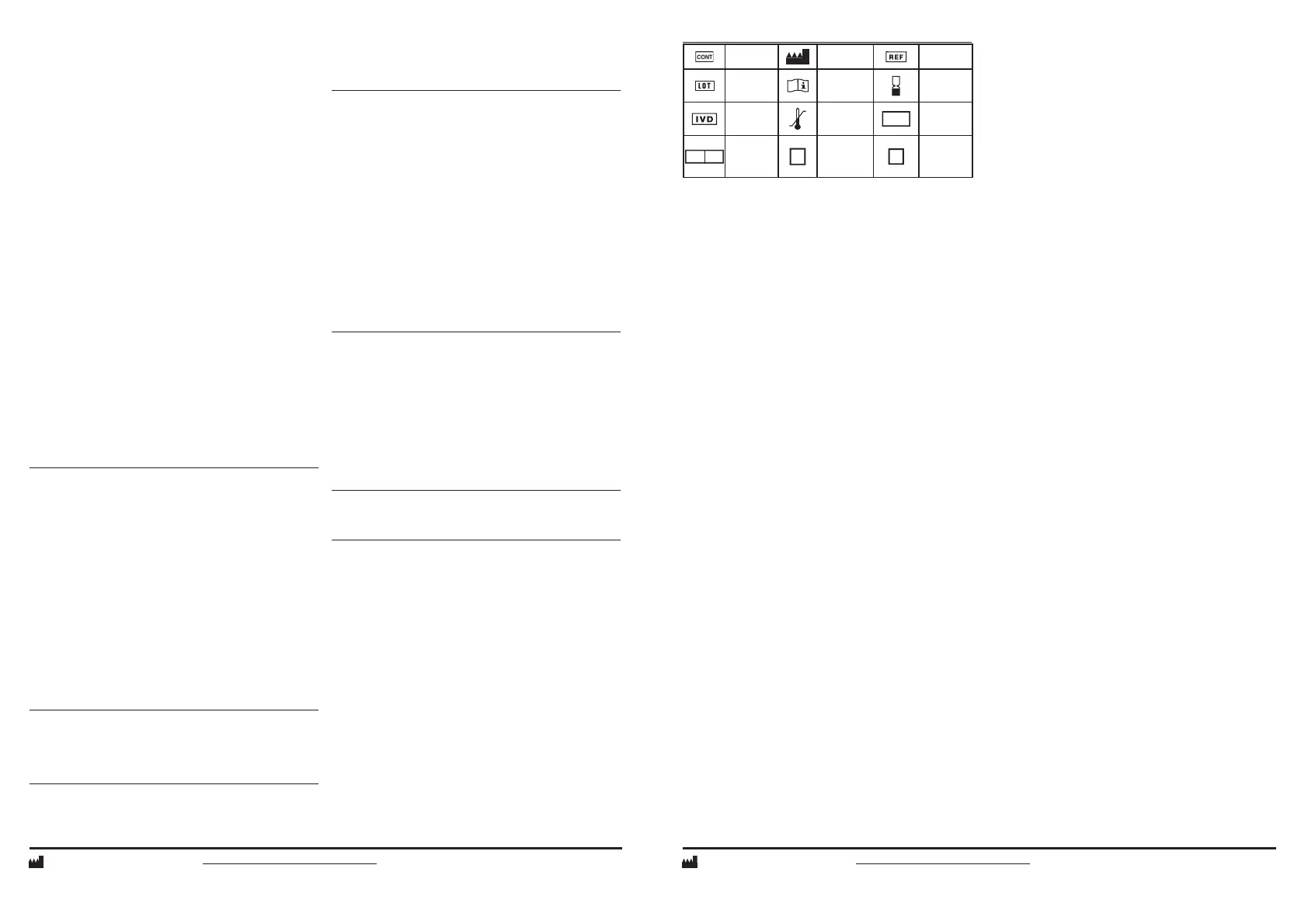
GLOSSARY OF SYMBOLS
Contents Manufacturer Catalog No.
Batch Code
See instruc-
tion for use
Use by
In vitro
diagnostic
device
Temperature
Limitation
OPENED
Date opened
/ Installation
date
STAB DAYS
Number
of days
onboard
stability
R1
Reagent 1
R2
Reagent 2
: Modification from previous version
Refer to the operator manual for additional information on installing
reagents and programming the analyzer, and running samples, calibra-
tors and controls.
CALIBRATION
Calibrate the instrument after loading new reagent, after maintenance
and whenever quality control results fall outside established limits. Under
typical use conditions, calibration factors for this test are valid for 7 days.
Refer to the operator manual for calibration procedures.
QUALITY CONTROL
Quality control requirements should be established in accordance with
local, state and/or federal regulations or accreditation requirements.
Assay at least two levels of serum control at least daily. Control materials
may be of human or animal origin, but should represent both clinically
normal and elevated levels of high density lipoprotein cholesterol.
Controls should also be assayed after maintaining the instrument, load-
ing a new reagent, and calibrating the analyzer.
CALCULATIONS
All calculations are performed by the instrument.
To calculate the result in SI units (mmol/L), multiply the result in
conventional units (mg/dL) by 0.0259.
LIMITATIONS / INTERFERING SUBSTANCES
- This method has not been certified by the Cholesterol Reference
Method Laboratory Network.
- Do not report results outside of the usable range.
- The results of this assay should only be interpreted in conjunction with
other diagnostic test results, clinical findings and the patient’s medical
history.
-
Lipemia may interfere with this test.
- Effects of icterus, hemolysis, and lipemia are estimated through the assay
of pools spiked with ditaurobilirubin, red blood cell hemolysate and Intralipid
®
20% solution. The effect of ascorbic acid was also tested. Observed biases
are shown below. Substances that affect results by more than both
3 mg/dL and 4% are reported as interfering substances in the Specimens
section.
Effects of Common Substances on HDL Cholesterol Recoveries
Interferant
Concentration Changes in Recoveries
Ascorbic Acid 3.0 mg/dL -0.3 at 57 mg/dL
†
Ditaurobilirubin 40 mg/dL* -0.7 at 57 mg/dL
‡
RBC hemolysate 200 mg/dL* -0.8 at 56 mg/dL
‡
Intralipid 20% solution 240 mg/dL* -3.3 at 52 mg/dL
400 mg/dL* -5.4 at 52 mg/dL
800 mg/dL* -1.5 at 54 mg/dL
2000 mg/dL* +2.5 at 54 mg/dL
* Refers to bilirubin, hemoglobin, and/or triglyceride concentration
† Effect is not statistically significant at α = 0.05.
‡ The observed effect is less than 3 mg/dL. This substance is not reported as
an interfering substance.
- Results may be falsely low when the sample is taken while levels of NAC,
NAPQI (a metabolite of acetaminophen (paracetamol)) or Metamizole are
significant.
- Many other substances can affect high density lipoprotein cholesterol
results. For additional information, refer to Effects of Drugs on Clinical
Laboratory Tests
7
and Effects of Preanalytical Variables on Clinical
Laboratory Tests.
8
PERFORMANCE CHARACTERISTICS
USABLE RANGE
The linear range of this assay is listed below. Specimens that exceed
the upper limit of this range should be diluted with normal saline and
reanalyzed. Multiply the results of diluted specimens by the appropriate
dilution factors.
Conventional Units SI Units
5 to 150 mg/dL 0.13 to 3.89 mmol/L
EXPECTED VALUES
The NCEP (American National Cholesterol Education Program) has
established the following classification for HDL cholesterol levels
according to the risk of developing coronary heart disease
4
:
Risk Classification Conventional Units SI Units
High risk < 40 mg/dL < 1.03 mmol/L
Low risk ≥ 60 mg/dL ≥ 1.55 mmol/L
LIMIT OF DETECTION
The limit of detection (LoD) for HDL cholesterol is 0.46 mg/dL, which
was determined based on the NCCLS protocol EP17-A
5
with proportions
of false positives (α) less than 5% and false negatives (β) less than 5%.
This LoD is based on 80 determinations, with 40 blank and 40 low level
samples, and LoB = 0.29 mg/dL.
ANALYTICAL SENSITIVITY
An absorbance change of 0.003 A on the Envoy 500 Chemistry System
corresponds to a change in HDL cholesterol concentration of approximately
1 mg/dL (0.03 mmol/L).
METHOD COMPARISON
One hundred and sixty serum and 152 plasma specimens were collected
from individual adult patients and assayed for high density lipoprotein
cholesterol using an Envoy 500 Chemistry System and another commer-
cially available method. Results were compared by least squares and
Passing - Bablok regression and the following statistics were obtained.
Serum/Plasma Comparison
n = 312 range = 5 to 158 mg/dL
Least Squares Regression
Envoy 500 = 0.7 mg/dL + 1.021 x Competitive Method
s
(y.x)
= 2.4 mg/dL r = 0.995
Passing - Bablok Regression
Envoy 500 = 0.7 mg/dL + 1.015 x Competitive Method
PRECISION
Two lipid controls were each assayed in triplicate twice per day over 8
days on an Envoy 500 Chemistry System. Estimates of within run and total
imprecision are calculated analogous to the methods described in NCCLS
publication EP3-T.
6
Precision of HDL Cholesterol Recoveries in mg/dL
Within Run Total
Sample n mean 1SD %CV 1SD %CV
Level 1 45 36.8 0.52 1.4% 0.72 2.0%
Level 2 48 71.1 0.68 1.0% 1.25 1.8%
REFERENCES
1. Badiman J J, et al. Regression of Atherosclerotic Lesions by High
Density Lipoprotein Plasma Fraction in the Cholesterol-Fed Rabbit.
Journal of Clinical Investigation 1990 85:1234-41.
2. Burtis C A, Ashwood E R, Eds. Tietz Textbook of Clinical Chemistry,
Third Edition W.B. Saunders Company: Philadelphia, PA, 1999.
3. Warnick G, et al., National Cholesterol Education Program
Recommendations for Measurement of High Density Lipoprotein
Cholesterol; Executive Summary Clin Chem, 41 10:1427 1995.
4. National Institutes of Health, National Cholesterol Education Program.
Detection Evaluation and Treatment of High Blood Cholesterol in Adults
(Adult Treatment Panel III), Final Report. NIH Publication No. 02-5215,
September 2002.
5. Protocols for the Determination of Limits of Detection and Limits of
Quantitation; Approved Guideline. NCCLS Document EP17-A. NCCLS,
Wayne PA, 2004.
6. Tentative Guidelines for Manufacturers for Establishing Performance
Claims for Clinical Chemical Methods, Replication Experiment NCCLS
Publication: Vol. 2 No. 20. Villanova, PA, 1982.
7. Young D S, Effects of Drugs on Clinical Laboratory Tests: Fifth Edition
AACC Press: Washington, DC, 2000.
8. Young D S, Effects of Preanalytical Variables on Clinical Laboratory
Tests: Second Edition AACC Press: Washington, DC, 1997.
Envoy is a registered trademark of ELITech Group.
.../...
(10/2015)
FTEVY-HDLL-v5

 Loading...
Loading...