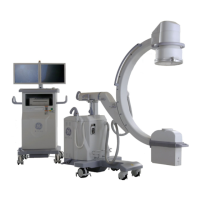
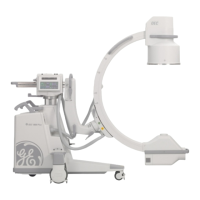
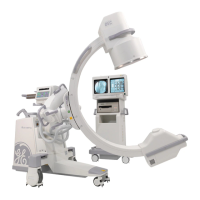
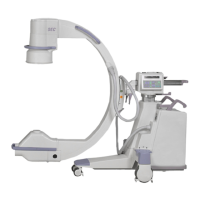
Introduction and Safety
15
26
1
Unique Device Identifier (UDI)
label(US only)
Every medical device has a unique
marking for identification.
Note that this is only an example of a
UDI marking.
The characters used in the UDI
marking represent specific
identifiers.
Device identifier:
(01)= GS1 global trade item
number (GTIN) of the device.
00001234567895 = Global trade
item number.
Production identifiers:
(11) = GS1 application identifier
for the manufacturing date of
the device.
150700= Manufacturing date:
year-month-day (YYMMDD) with
DD always equal to 00.
(21) = GS1 application identifier
for the serial number of the
device.
AAAAAYY00001AA = Serial
27
1
This label indicates the system
was tested by ITS authorized
body and was found to be in
compliance with the
requirements of all relevant
directives and standards at the
time of manufacture.
28
1
This label can be found on the X-
Ray generator. It indicates the
location of the X-Ray source and
the controls used to produce
ionizing X-Radiation. Use
appropriate precautions and
protective equipment when using
the system and at all time when
X-Rays are present.

 Loading...
Loading...