1
INSTRUCTIONS FOR USE FOR:
GORE® CARDIOFORM Septal Occluder
Carefully read all instructions prior to use. Observe all warnings and
precautions noted throughout these instructions.
Failure to do so may result in complications.
DESCRIPTION
The GORE® CARDIOFORM Septal Occluder consists of an implantable Occluder
and a Delivery System. The Occluder is comprised of a platinum-filled nickel-
titanium (Nitinol) wire frame covered with expanded polytetrafluoroethylene
(ePTFE). The ePTFE includes a hydrophilic surface treatment to facilitate
echocardiographic imaging of the Occluder and surrounding tissue during
implantation. When fully deployed, the Occluder assumes a double-disc
configuration to prevent shunting of blood between the right and left atria. The
Delivery System consists of a 75 cm working length 10 Fr outer diameter Delivery
Catheter that is coupled to a Handle. The Handle facilitates loading, deployment,
and locking of the Occluder. The Handle also allows repositioning and retrieval of
the Occluder via the Retrieval Cord, if necessary.
The Occluder is available in diameters of 15, 20, 25, and 30 mm. The Occluder is
delivered using conventional catheter delivery techniques and may be delivered
with the aid of a 0.035" guidewire (or smaller), if desired.
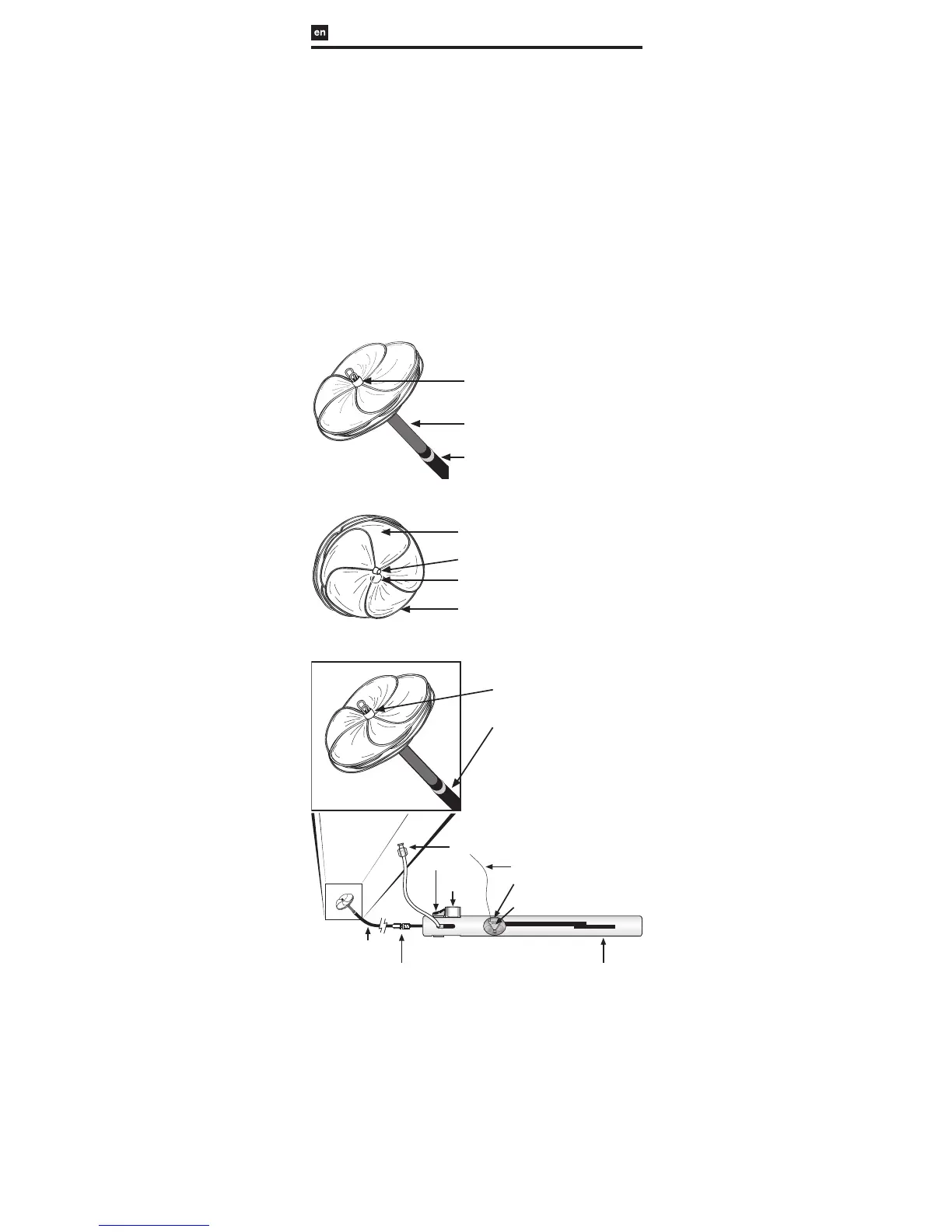
FIGURE 1: GORE® CARDIOFORM Septal Occluder
FIGURE 1a: Left Atrial View
Left Atrial Eyelet
Control Catheter (Gray)
Delivery Catheter (Blue)
FIGURE 1b: Right Atrial View
Occluder Leaflet
Platinum-Filled
Nitinol Wire Frame
Lock Loop
Right Atrial Eyelet
FIGURE 2: GORE® CARDIOFORM Septal Occluder Delivery System
Left Atrial Eyelet
Flush Port
Packaging
Insert (clear)
Occluder Lock
(red)
Delivery Catheter (Blue)
Retrieval Cord
Slider (gray)
Retrieval Cord Lock (red)
Handle
Retrieval Luer
Delivery Catheter (blue)
- 75 cm Working Length -
INDICATIONS / INTENDED USE
The GORE® CARDIOFORM Septal Occluder is a permanently implanted device
indicated for the percutaneous, transcatheter closure of ostium secundum atrial
septal defects (ASDs).
CONTRAINDICATIONS
The GORE® CARDIOFORM Septal Occluder is contraindicated for use in patients:
• Unable to take anti-platelet or anticoagulant medications such as aspirin,
heparin, or warfarin.
• With anatomy where the GORE® CARDIOFORM Septal Occluder size or
position would interfere with other intracardiac or intravascular structures,
such as cardiac valves or pulmonary veins.
• With active endocarditis, or other infections producing bacteremia, or
patients with known sepsis within one month of planned implantation,
or any other infection that cannot be treated successfully prior to device
placement.
• With known intracardiac thrombi.
 Loading...
Loading...