Table 8: Type 1 Diabetes Mellitus–Adult (continued)
Study A Study B Study C
Treatment duration
Treatment in
combination with
28 weeks
Regular insulin
28 weeks
Regular insulin
16 weeks
Insulin lispro
LANTUS NPH LANTUS NPH LANTUS NPH
HbA1c
Baseline HbA1c 8.0 8.0 7.7 7.7 7.6 7.7
Adj. mean change from
baseline
+0.2 +0.1 -0.2 -0.2 -0.1 -0.1
LANTUS – NPH +0.1 +0.1 0.0
95% CI for Treatment
difference
(0.0; +0.2) (-0.1; +0.2) (-0.1; +0.1)
Basal insulin dose
Baseline mean 21 23 29 29 28 28
Mean change from
baseline
-2 0 -4 +2 -5 +1
Total insulin dose
Baseline mean 48 52 50 51 50 50
Mean change from
baseline
-1 0 0 +4 -3 0
Fasting blood glucose
(mg/dL)
Baseline mean 167 166 166 175 175 173
Adj. mean change from
baseline
-21 -16 -20 -17 -29 -12
Body weight (kg)
Baseline mean 73.2 74.8 75.5 75.0 74.8 75.6
Mean change from
baseline
0.1 -0.0 0.7 1.0 0.1 0.5
Type 1 Diabetes–Pediatric (see Table 9).
In a randomized, controlled clinical study (Study D), pediatric patients (age range 6 to 15 years) with
type 1 diabetes (n=349) were treated for 28 weeks with a basal-bolus insulin regimen where regular
human insulin was used before each meal. LANTUS was administered once daily at bedtime and NPH
insulin was administered once or twice daily. Similar effects on HbA1c (Table 9) and the incidence of
hypoglycemia were observed in both treatment groups
[See Adverse Reactions (6.1)].
Table 9: Type 1 Diabetes Mellitus–Pediatric
Study D
Treatment duration 28 weeks
Treatment in combination with Regular insulin
LANTUS NPH
Number of subjects treated 174 175
HbA1c
Baseline mean 8.5 8.8
Adj. mean change from baseline +0.3 +0.3
LANTUS – NPH 0.0
95% CI for Treatment difference (-0.2; +0.3)
Basal insulin dose
Baseline mean 19 19
Mean change from baseline -1 +2
Total insulin dose
Baseline mean 43 43
Mean change from baseline +2 +3
Fasting blood glucose (mg/dL)
Baseline mean 194 191
Adj. mean change from baseline -23 -12
Body weight (kg)
Baseline mean 45.5 44.6
Mean change from baseline 2.2 2.5
Type 2 Diabetes–Adult (see Table 10).
In a randomized, controlled clinical study (Study E) (n=570), LANTUS was evaluated for 52 weeks in
combination with oral anti-diabetic medications (a sulfonylurea, metformin, acarbose, or combinations
of these drugs). LANTUS administered once daily at bedtime was as effective as NPH insulin
administered once daily at bedtime in reducing HbA1c and fasting glucose (Table 10). The rate of
hypoglycemia was similar in LANTUS and NPH insulin treated patients
[See Adverse Reactions (6.1)].
In a randomized, controlled clinical study (Study F), in patients with type 2 diabetes not using oral
anti-diabetic medications (n=518), a basal-bolus regimen of LANTUS once daily at bedtime or NPH
insulin administered once or twice daily was evaluated for 28 weeks. Regular human insulin was used
before meals, as needed. LANTUS had similar effectiveness as either once- or twice-daily NPH insulin
in reducing HbA1c and fasting glucose (Table 10) with a similar incidence of hypoglycemia
[See
Adverse Reactions (6.1)].
In a randomized, controlled clinical study (Study G), patients with type 2 diabetes were randomized to
5 years of treatment with once-daily LANTUS or twice-daily NPH insulin. For patients not previously
treated with insulin, the starting dose of LANTUS or NPH insulin was 10 units daily. Patients who were
already treated with NPH insulin either continued on the same total daily NPH insulin dose or started
LANTUS at a dose that was 80% of the total previous NPH insulin dose. The primary endpoint for this
study was a comparison of the progression of diabetic retinopathy by 3 or more steps on the Early
Treatment Diabetic Retinopathy Study (ETDRS) scale. HbA1c change from baseline was a secondary
endpoint. Similar glycemic control in the 2 treatment groups was desired in order to not confound the
interpretation of the retinal data. Patients or study personnel used an algorithm to adjust the LANTUS
and NPH insulin doses to a target fasting plasma glucose ≤100 mg/dL. After the LANTUS or NPH
insulin dose was adjusted, other anti-diabetic agents, including pre-meal insulin were to be adjusted
or added. The LANTUS group had a smaller mean reduction from baseline in HbA1c compared to the
NPH insulin group, which may be explained by the lower daily basal insulin doses in the LANTUS group
(Table 10). Both treatment groups had a similar incidence of reported symptomatic hypoglycemia. The
incidences of severe symptomatic hypoglycemia are given in Table 6
[See Adverse Reactions (6.1)].
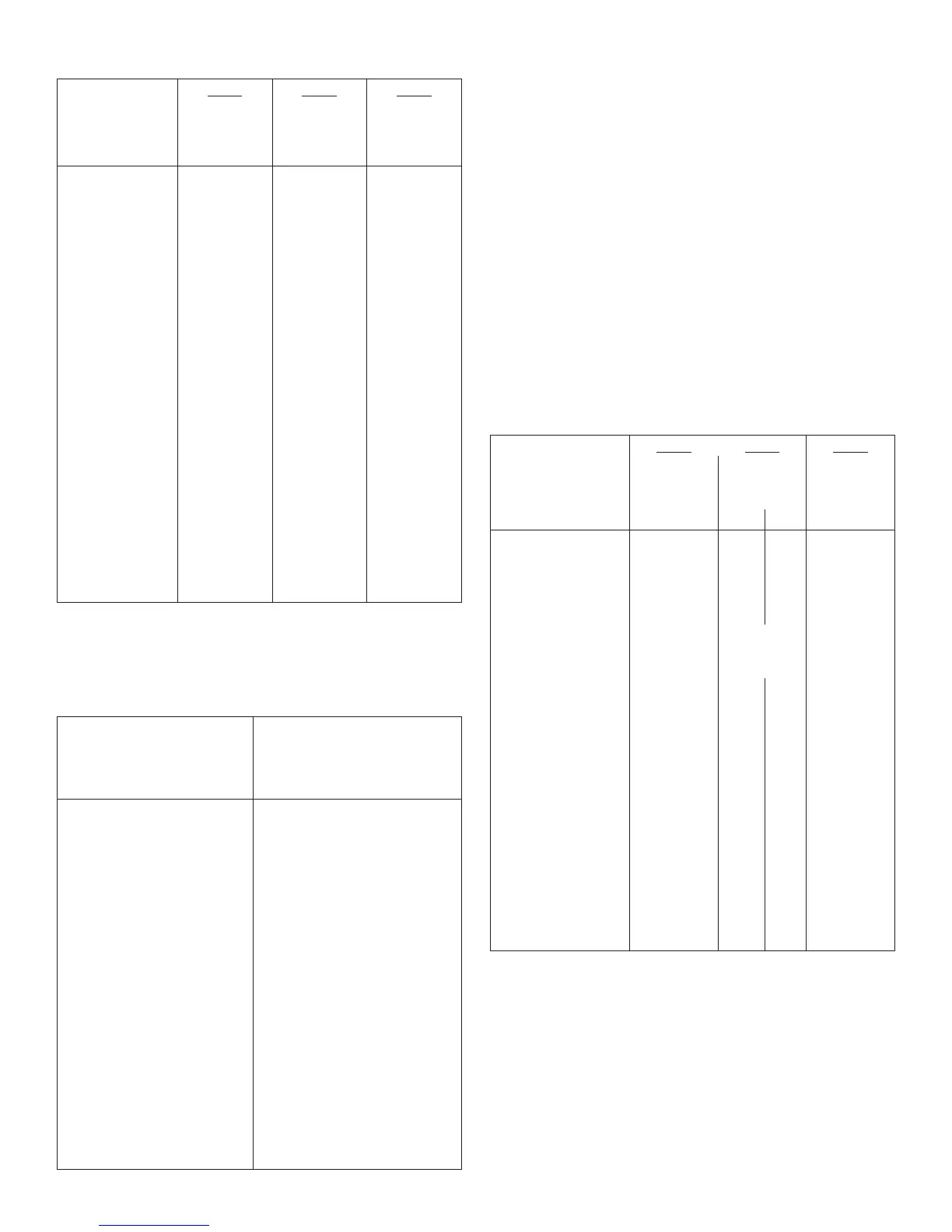
Table 10: Type 2 Diabetes Mellitus–Adult
Study E Study F Study G
Treatment duration 52 weeks 28 weeks 5 years
Treatment in combination
with
Oral agents Regular insulin Regular insulin
LANTUS NPH LANTUS NPH LANTUS NPH
Number of subjects treated 289 281 259 259 513 504
HbA1c
Baseline mean 9.0 8.9 8.6 8.5 8.4 8.3
Adj. mean change from
baseline
-0.5 -0.4 -0.4 -0.6 -0.6 -0.8
LANTUS – NPH -0.1 +0.2 +0.2
95% CI for Treatment
difference
(-0.3; +0.1) (0.0; +0.4) (+0.1, +0.4)
Basal insulin dose
*
Baseline mean 14 15 44.1 45.5 39 44
Mean change from baseline +12 +9 -1 +7 +23 +30
Total insulin dose
*
Baseline mean 14 15 64 67 48 53
Mean change from baseline +12 +9 +10 +13 +41 +40
Fasting blood glucose (mg/dL)
Baseline mean 179 180 164 166 190 180
Adj. mean change from
baseline
-49 -46 -24 -22 -45 -44
Body weight (kg)
Baseline mean 83.5 82.1 89.6 90.7 100 99
Adj. mean change from
baseline
2.0 1.9 0.4 1.4 3.7 4.8
*In Study G, the baseline dose of basal or total insulin was the first available on-treatment dose
prescribed during the study (on visit month 1.5).
LANTUS Timing of Daily Dosing (see Table 11).
The safety and efficacy of LANTUS administered pre-breakfast, pre-dinner, or at bedtime were
evaluated in a randomized, controlled clinical study in patients with type 1 diabetes (study H, n=378).
Patients were also treated with insulin lispro at mealtime. LANTUS administered at different times of
the day resulted in similar reductions in HbA1c compared to that with bedtime administration (see Table
11). In these patients, data are available from 8-point home glucose monitoring. The maximum mean
blood glucose was observed just prior to injection of LANTUS regardless of time of administration.
In this study, 5% of patients in the LANTUS-breakfast arm discontinued treatment because of lack of
efficacy. No patients in the other two arms discontinued for this reason. The safety and efficacy of
LANTUS administered pre-breakfast or at bedtime were also evaluated in a randomized, active-
controlled clinical study (Study I, n=697) in patients with type 2 diabetes not adequately controlled on
oral anti-diabetic therapy. All patients in this study also received glimepiride 3 mg daily. LANTUS given
before breakfast was at least as effective in lowering HbA1c as LANTUS given at bedtime or NPH
insulin given at bedtime (see Table 11).
5
 Loading...
Loading...