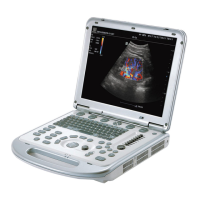
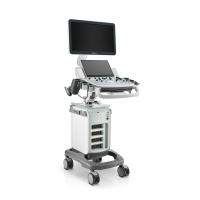
Operator’s Manual 2 - 1
2 System Overview
2.1 Intended Use
Consona N9 series Diagnostic Ultrasound System is applicable for adults, pregnant women,
pediatric patients and neonates.It is intended for use in fetal, abdominal, Intra-operative, pediatric,
small organ(breast, thyroid, testes), neonatal cephalic,adult cephalic,trans-rectal, trans-vaginal,
musculo-skeletal(conventional), musculo- skeletal(superficial), Thoracic/Pleural, cardiac adult,
cardiac pediatric, Trans-esoph., peripheral vessel and urology exams.
Consona N9 series Diagnostic Ultrasound System Modes of operation include: B, M, PWD, CWD,
Color Doppler, Amplitude Doppler, Combined mode(B+M, PW+B, Color+B, Power+B,
PW+Color+B, Power+PW+B), Tissue Harmonic Imaging, Smart 3D, 4D(Real-time 3D), iScape
View, TDI, Color M, Strain Elastography, STE, STQ, Contrast imaging (Contrast agent for LVO),
Contrast imaging (Contrast agent for Liver).
This device is a general purpose diagnostic ultrasound system intended for use by qualified and
trained healthcare professionals for ultrasound imaging, measurement, display and analysis of the
human body and fluid, which is intended to be used in a hospital or medical clinic.
2.2 Contraindication
The diagnostic ultrasound system is not intended for ophthalmic use.
2.3 Safety Classifications
• According to the type of protection against electric shock:
Externally powered Class I equipment + internally powered equipment
• According to the degree of protection against electric shock:
Type-BF&CF applied part. The ECG is type-CF applied part. The PCG and ultrasound probes
are type-BF applied parts.
• According to the degree of protection against harmful ingress of water:
– The main unit is rated as IPX0
– The probes are rated as IPX7
– The foot switch (can be applied in the operating room) is rated as IPX8
• According to the disinfection and sterilization method(s) recommended by manufacturer:
Equipment with disinfection and sterilization method(s) recommended by manufacturer.
• According to the degree of safety of application in the presence of a FLAMMABLE
ANESTHETIC MIXTURE WITH AIR or WITH OXYGEN OR NITROUS OXIDE
EQUIPMENT not suitable for use in the presence of a FLAMMABLE ANESTHETIC
MIXTURE WITH AIR or WITH OXYGEN OR NITROUS OXIDE
• According to the signal input and output parts of the device:
The device is equipped with signal input and output parts
• According to the mode of operation:
Continuous operation

 Loading...
Loading...