Regulatory Information
Safety Standards & Specifications 10-5
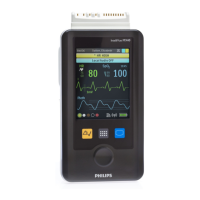
The MX40 can provide time-limited local monitoring when it is not connected
to the wireless network.
Unlike a traditional bedside monitor which operates on AC power, the MX40
is powered by battery and cannot provide continuous monitoring.
Authorized EU Representative
Philips Medizin Systeme Deutschland
Hewlett-Packard-Strasse 2
D 71034, Boeblingen
Germany
Authorized Australia Sponsor
Philips Electronics Australia
65 Epping Road
North Ryde NSW, Australia 2113
Patient Population
This device is not for use with infant or neonatal patients.
Clinical judgment must be used to determine when the MX40 should be used
on a specific pediatric patient, as it is not possible to assign a precise weight
or age to ECG performance.
Use of the device is restricted to one patient at a time.
The components/accessories which come into contact with the patient’s skin
are in compliance with the relevant requirements of EN ISO 10993-1 for
Biocompatibility. The device is not designed for direct contact with the
patient’s skin. The accompanying pouch is the appropriate means for holding
the device.
Rx
Federal Law restricts this device to sale by or on the order of a physician.
Contraindications
A Contraindication describes a situation, such as patient population,
medical reason, or clinical condition, in which a device may not be used
because the risk of use clearly outweighs any possible benefit.
There are no contraindications for use of the MX40.
 Loading...
Loading...