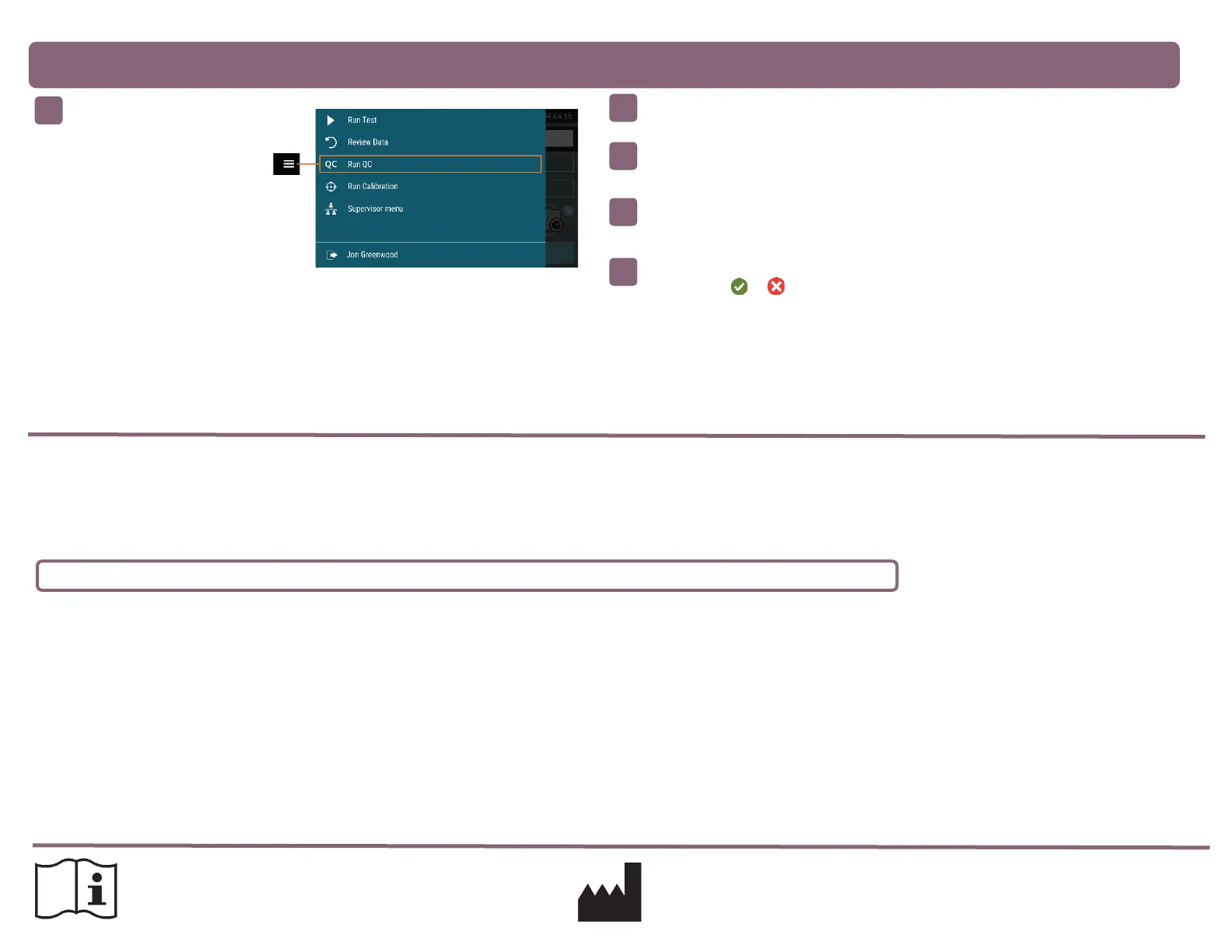
Follow the External Quality Control Test Procedure in the Package Insert to test each
Control, first the Positive Control followed by the Negative Control.
Quidel recommends that Positive and Negative External Controls be run:
▪
Once for each untrained operator.
▪
Once for each new shipment of kits – provided that each different lot received in the shipment is tested.
▪
As deemed additionally necessary by your internal quality control procedures, and in accordance with Local, State and Federal regulations or accreditation
requirements.
Intended Use
Sofia 2 Campylobacter FIA employs immunofluorescence for the rapid qualitative detection of a Campylobacter-specific antigen in human fecal specimens. Sofia 2
Campylobacter FIA is designed to detect C. jejuni, C. coli, C. lari and C. upsaliensis from patients with signs and symptoms of gastroenteritis. The test is intended for use
with preserved fecal specimens in transport media and unpreserved fecal specimens. Test results should be considered in conjunction with clinical findings and patient
history.
ASSISTANCE
If you have any questions regarding the use of this product or if you want to report a test system problem, please call Quidel Technical Support 800.874.1517 (in the
U.S.), 858.552.1100 (outside the U.S.), technicalsupport@quidel.com, or your local distributor.
Reference the Package Insert for Warnings and Precautions, Specimen Collection and Handling, and Quality Control.
Study the Package Insert and User Manual thoroughly
before using Quick Reference Instructions. This is not a
complete Package Insert.
 Loading...
Loading...