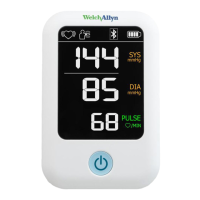
18 Semi-Automatic Blood Pressure Monitor Welch Allyn
Reference to standards
Technical specifications
Device standard: Device corresponds to the requirements of the
European standard for non-invasive blood
pressure monitor
EN1060-1/12:95
EN 1 060-3/09:97
DIN 58130, NIBP - clinical investigation
ANSI/AAMI SP10, NIBP - requirements
Electromagnetic
compatibility:
Device fulfils the stipulations of the European
standard EN 60601-1-2
Clinical testing: The clinical performance test was carried out in
Germany according to the DIN 58130/1997
procedure N6 (sequential).
The stipulations of the EU-Guidelines 93/42/
EWG for Medical Products Class IIa have been
fulfilled.
Size: 125 x 155 x 80 mm
Weight: 265 g (with batteries)
Storage temperature: -5 to +50°C
Humidity: 15 to 85% relative humidity maximum
Operation temperature: 10 to 40°C
Display: LCD-Display (Liquid Crystal Display)
Measuring method: Oscillometric
Pressure sensor: Capacitive
 Loading...
Loading...