Page 7 of 9
10.1.1 Equipment required
Alcohol Wipe –Isopropyl Alcohol 70% by Vol.
Protective Clothing Safety Note – Always refer to the Health & Safety Data Sheet associated to the Wipes for
appropriate protective clothing before using.
Drying Cloth - A clean, disposable, absorbent, non-shedding cloth or hot-air dryer
A First Aid kit and eyewash bottle - In case of splashing with Alcohol Wipe.
10.1.2 Procedure for a Cry-Ac
®
, Cry-Ac-3
®
or Cry-Baby
®
Safety Precaution: Ensure the Cry-Ac
®
, Cry-Ac-3
®
, or Cry-Baby
®
is empty of Liquid Nitrogen before
commencing cleaning. Refer to Section 4 to depressurize the Unit and refer to the MSDS for the disposal
of any remaining Liquid Nitrogen.
Wear appropriate protective clothing and ensure that all outer surfaces are thoroughly wiped.
Periodically change the alcohol wipe until all surfaces have been cleaned.
Ensure all surfaces are carefully hand-dried using a dry cloth or industrial hot-air dryer.
Safely dispose of cleaning materials.
The Cry-Ac devices should no longer be used when the exterior of bottle is frosted over. This indicates the
vacuum inside the bottle has deteriorated due to age or the bottle has been damaged by the end user.
11. Recommended methods of Sterilization, Temperature and times.
If the product is intended to be used in the sterile field the device must be sterilized. Sterilize the Cry-Ac
®
, Cry-Ac-3
®
, or
Cry-Baby
®
using the recommended validated parameters below:
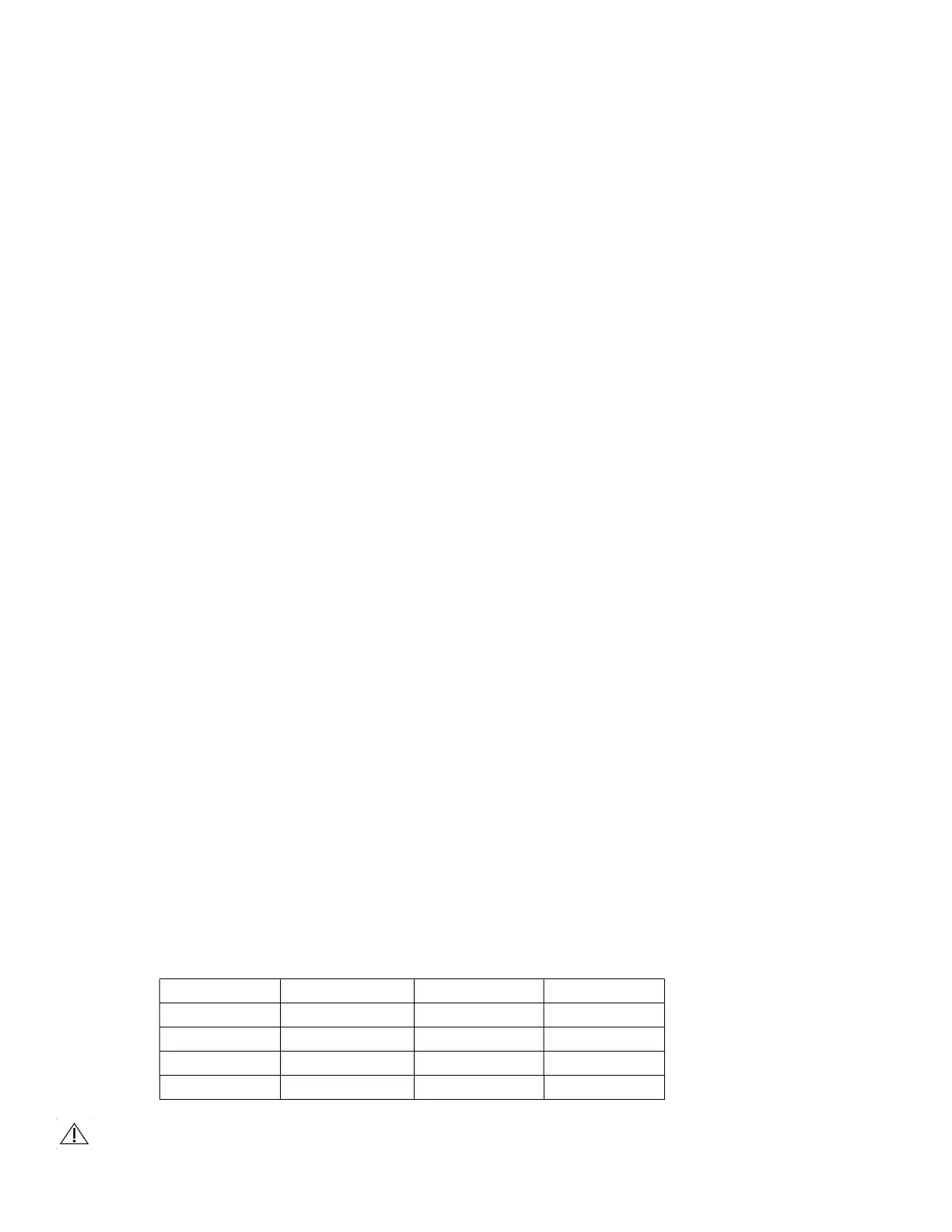
i) Use the following recommended validated sterilization parameters: of sterilization.
Moist heat sterilization with Gravity cycle is the recommended method of sterilization.
Vaporized Hydrogen (VHP), Ethylene oxide (EO), gas plasma and dry heat are not recommended
sterilization methods for reusable instruments.
The recommended parameters demonstrate the minimum validated steam sterilization time and
temperature required to achieve a 1.0 x 10-6 sterility assurance level (SAL)
Sterilize the instruments after placing them in a Stainless-Steel Sterilization Tray and wrapping
the tray in a double layer of Bioshield Sterilization Wrap using the envelope technique. Use a
FDA cleared sterilization tray and/or wrap for this purpose
The validated reprocessing instructions are not applicable to trays that include devices not
manufactured or distributed by Brymill.
Damage can occur due to improper reprocessing procedures
Cycle Time Temperature Exposure Time Dry Time
Gravity 121*C (250*F) 30 15
 Loading...
Loading...