18
The information on the unit’s capabilities provided by the manufacturer in accordance with EN
13544-1 may not apply to medicines provided in suspension form or those that are highly viscous.
EN 60601-1 Medical electrical equipment – Part 1: general requirements for safety
EN 60601-1-2 Medical electrical equipment – Part 1: General requirements for basic safety and es-
sential performance. Collateral standard: Electromagnetic compatibility - Requirements and tests.
EN 13544-1 Respiratory therapy equipment – Part one: Nebulising systems and their components.
This section contains information specic to product compliance with the EN 60601-1-2 stand-
ard. The Chicco aerosol Super Soft is a medical electrical device that requires special precautions
regarding electromagnetic compatibility and needs to be installed and commissioned accord-
ing to the electromagnetic information provided.
Mobile and portable RF communications equipment (mobile phones, transceivers, etc.) may
aect the device.
The Chicco aerosol Super Soft is intended for use in the electromagnetic environment specied below.
Customers or users of the product must ensure that it is used in the recommended environment.
Chicco nebulizers Super Soft 00009067000000 and 00009813000000 is intended for
use in the electromagnetic environment specied below. The customer or the user of Chicco
nebulizers 00009067000000 and 00009813000000 must make sure that it is used in such
an environment.
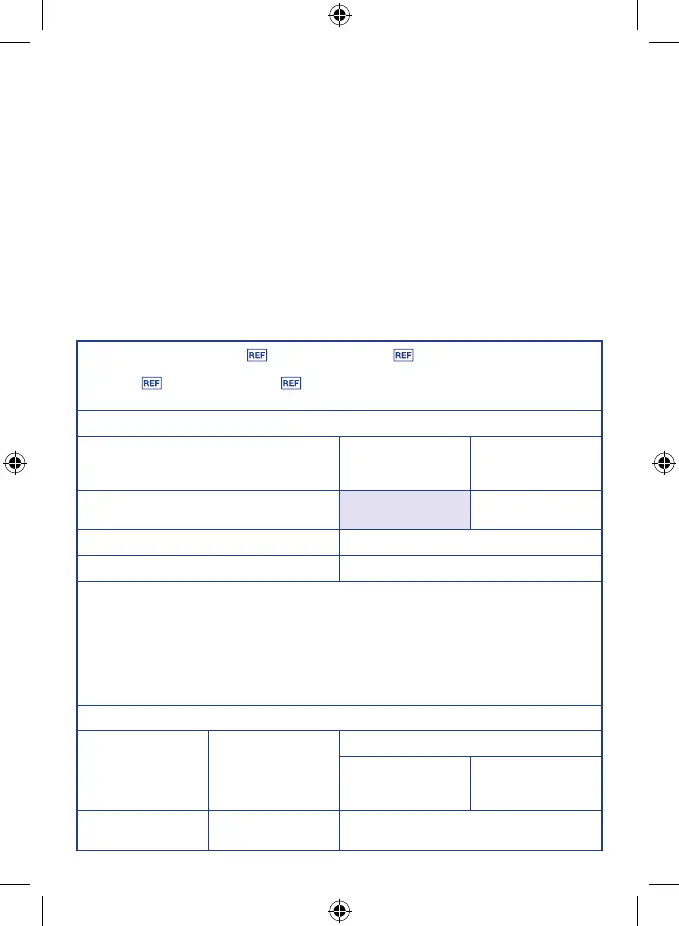
Guidance and manufacturer’s declaration - Electromagnetic emissions
Phenomenon
Professional
healthcare facility
environment
a
)
HOME HEALTHCARE
ENVIRONMENT
a
)
Conducted and radiated RF EMISSIONS
a
)
CISPR 11
Group 1 Class B
Harmonic distortion IEC 61000-3-2
b
) Class A
Voltage uctuations and ickering IEC 61000-3-3
b
) COMPLIANT
a
) The equipment is suitable for use in Home Health Environments and Professional Health Care
Environments limited to patient rooms and respiratory treatment facilities in hospital or clinics.
The more restrictive acceptance limits of Group 1 Class B (CISPR 11) have been considered
and applied. The equipment is suitable for use in the mentioned environments when directly
connected to the Public Mains Network.
b
) The test is not applicable in this environment unless the ME EQUIPMENT and ME SYSTEM used
will be connected to the PUBLIC MAINS NETWORK and the power input is otherwise within the
scope of the Basic EMC standard.
Guidance and manufacturer’s declaration - Electromagnetic immunity - Enclosure port
Phenomenon
Basic EMC standard
or test method
Immunity test levels
Professional
healthcare facility
environment
Home healthcare
environment
ELECTROSTATIC
DISCHARGE
IEC 61000-4-2
± 8kV contact
± 2 kV, ±4kV, ±8 kV, ±15 kV air
 Loading...
Loading...