18
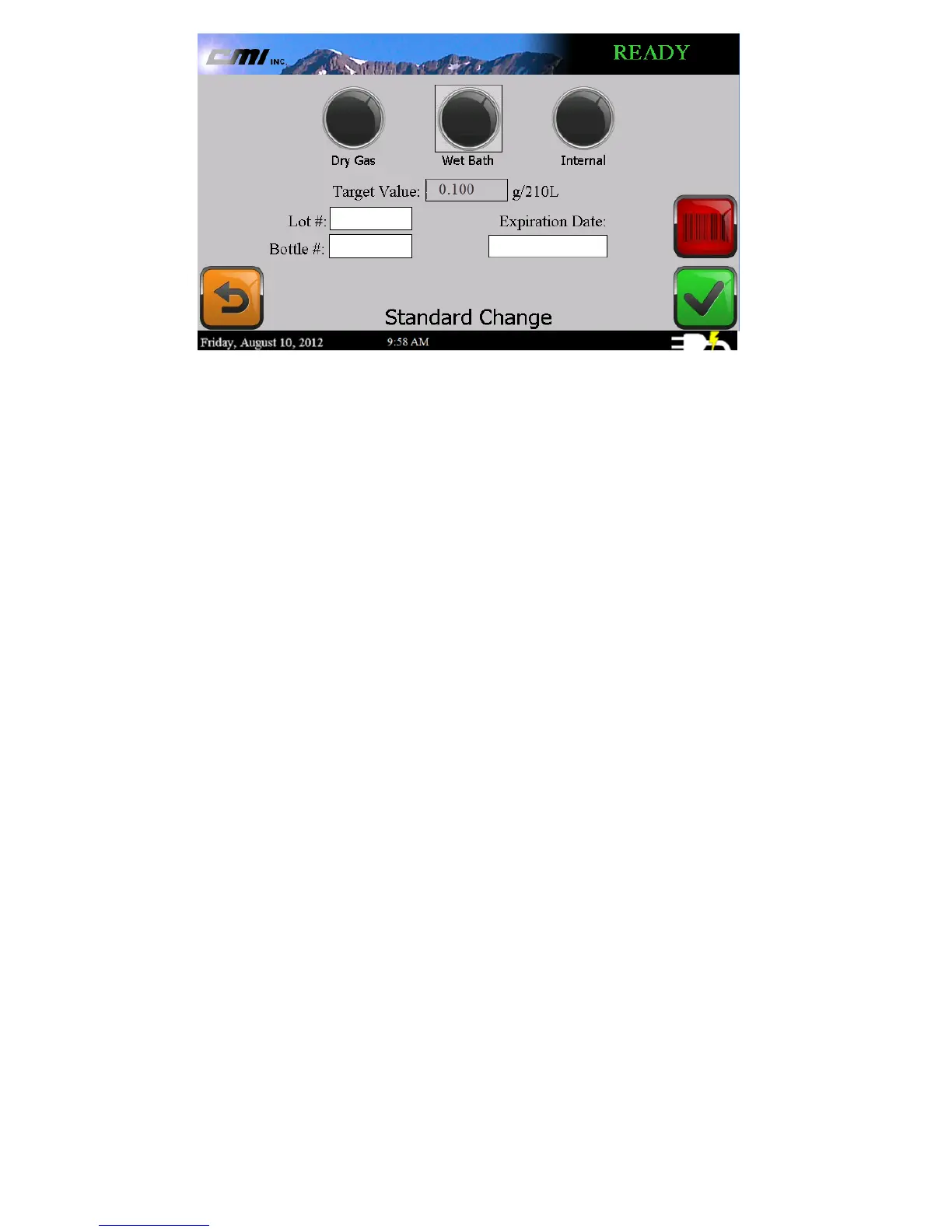
5. Using the information from the label on the solution
bottle, enter the following fields on the Standard
Change screen:
a. The Lot #.
b. The Bottle #.
c. The Expiration Date.
6. Select the check button to begin the I-9000 solution
change stability test.
The I-9000 will include the solution change record as
part of the Intoxilyzer Performance Report (IPR)
printed with each subject’s EBAT.
7. Review the stability test results for acceptability.
NOTE: All 10 calibration checks performed must fall
within the 0.095 to 0.105 g/210L in order to pass.
Any result outside of the acceptable range requires
the solution change to be repeated.
8. Once completed, select the BACK button until the
I-9000 returns to the main screen.
 Loading...
Loading...