1
Instructions for Use
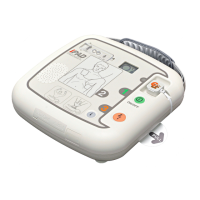
i-PAD CU-SP1 AUTO
The information in these Instructions for Use applies to the i-PAD CU-SP1 AUTO. This
information is subject to change. Please contact CU Medical Systems, Inc. or its authorized
representatives for information on revisions.
Revision History
Edition1
Publication Date: March 2014
Document No.: SPA-OPM-E-01
Published by: CU Medical Systems, Inc.
Printed in the Korea
Copyright
© 2014 CU Medical Systems, Inc.
No part of these Instructions for Use may be reproduced without the permission of CU
Medical Systems, Inc.
Medical Device Directive
The i-PAD CU-SP1 AUTO complies with the requirements of the Medical Device Directive
2007/47/EC and its revisions.
Important:
Quick defibrillation is needed if sudden cardiac arrest occurs. Since the chance of success is
reduced by 7% to 10% for every minute that defibrillation is delayed, defibrillation must be
performed promptly.