4
WARNINGS (continued)
• Device is designed to comply with electromagnetic safety standards. This equipment generates, uses, and can radiate radio frequency energy and, if not installed and
used in accordance with instructions, may cause harmful interference to other devices in the vicinity. However, there is no guarantee that interference will not occur in a
particular installation. Harmful interference to other devices can be determined by turning this equipment on and o. Try to correct the interference using one or more of
the following:
• Reorient or relocate the receiving device
• Increase the separation between the equipment
• Consult your local Customer Service representative for help
• Care must be taken when operating this equipment around other equipment to avoid reciprocal interference. Potential electromagnetic or other interference could occur
to this or to the other equipment. Try to minimize this interference by not using other equipment in conjunction with this device.
• Ensure the pump control unit is turned o and unplugged from the wall outlet prior to and while cleaning or disinfecting.
• Equipment should not be used in the presence of any ammable anesthetic mixture with air, oxygen, or nitrous oxide.
• Contains no user serviceable parts. Contact your local Customer Service representative.
• Do not place any items in an autoclave.
• No Service is to be attempted while the device is in use.
• This device is NOT to be altered or modied.
CAUTIONS
• Medical Electrical Equipment needs special precautions regarding EMC. Portable and mobile RF communication equipment can be aected by other medical electrical
devices. If you believe interference is occurring, please consult Electromagnetic Compatibility (EMC) section.
• To prevent extremity compartment syndrome, special attention should be given to patients who are positioned in the supine lithotomy position for extended lengths of
time. This includes patients with or without cus.
• Cus used in combination with warming devices may cause skin irritation. Regularly check for patient discomfort, compliance, and skin irritation.
• Allow cus to warm to room temperature if exposed to temperatures below 5⁰C (41⁰F).
• Do not immerse in any liquid for any reason.
• Do not operate device in a wet environment.
• Equipment should be used in a lint-free and dust-free environment.
• Do not subject the unit to extreme shocks, such as dropping the pump.
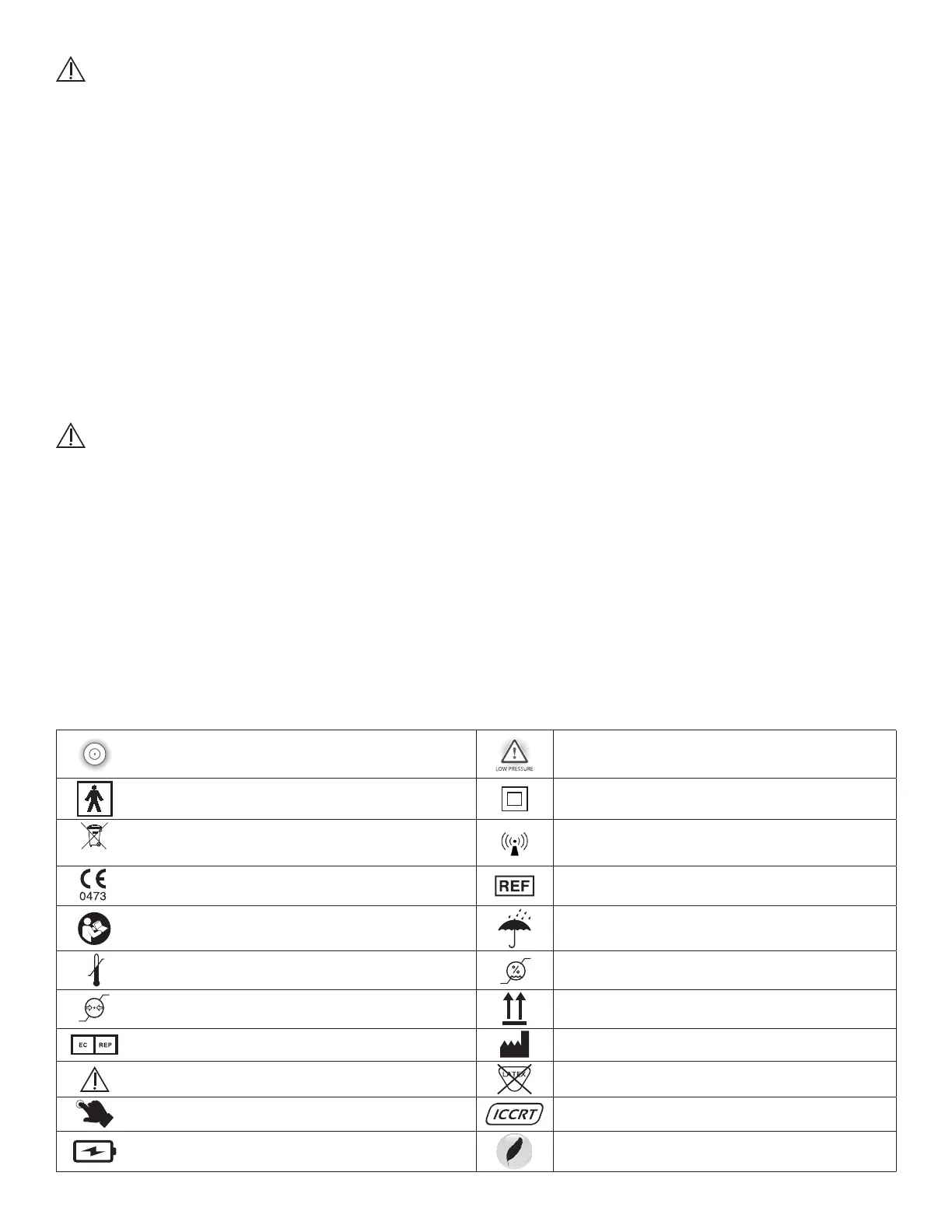
SYMBOLS
Power button and battery indicator
Low pressure indicator
This symbol designates the degree of protection against electrical shock from
the wrap as being a type BF applied part
Class II medical electrical equipment
Waste of electrical and electronic equipment must not be disposed as unsorted munici-
pal waste and must be collected separately. Contact an authorized representative of the
manufacturer for information concerning the decommissioning of your equipment.
The use of accessories, power supplies and cables other than those specied, with the
exception of components sold by the manufacturer of the VenaPro as replacement
parts, may result in increased emissions or decreased immunity of the VenaPro
CE Mark of conformity with notied body number Catalogue number
Refer to Instruction Manual/Booklet Keep dry
Temperature range Humidity range
Atmospheric pressure range This end up
Authorized repesentative in the European Community Manufacturer with 4-digit year of manufacture printed underneath
Warning or Caution
Not made with natural rubber latex
One touch operation Integrated cool care releasing technology
Battery operated Lightweight
 Loading...
Loading...