23
Procedure:
-
F
ill a container of a suitable size to hold all the individual components to be disinfected with a solution of drinking water and disin-
fectant, observing the proportions indicated on the packaging of the disinfectant.
-
C
ompletely immerse each individual component in the solution, taking care to avoid the formation of air bubbles in contact with
the components. Leave the components immersed for the period of time indicated on the disinfectant packaging, and associated
with the concentration chosen for the preparation of the solution.
-
R
ecover the disinfected components and rinse them thoroughly with lukewarm drinking water.
-
A
fter disinfecting the accessories, shake them vigorously and place them on a paper towel, or alternatively dry them with a hot air
jet (e.g. hair dryer).
-
Dispose of the solution ac
cording to the disinfectant manufacturer's instructions.
Sterilisation
The sterilisation procedure described in this section is only eective on treated components if it is adhered to in all points and only if
the components to be treated are sanitised beforehand, and is validated in accordance with ISO 17665-1.
Equipment: Steam steriliser with fractionated vacuum and overpressure compliant with EN 13060.
Procedure: Package each individual component to be treated in sterile barrier system or packaging in accordance with EN 11607.
Insert the packed components into the steam steriliser, making sure to keep the vessel (9) upright.
Perform the sterilisation cycle in accordance with the instructions for use of the equipment by selecting a temperature of:
(method A):
134°C and a time of 10 minutes rst.
(method B): 121°C and a time of 20 minutes rst.
Storage:
After sanitising, disinfection or sterilisation, reassemble the vessel and connecting pipes according to the instructions given in the
'CONNECTION DIAGRAM'.
At the end of each use, store the medical device complete with accessories in a dry and dust-free place.
• The manual aspirated ow control (15) is a sterile disposable product and must be replaced each time it is used.
•
T
he ANTIBACTERIAL HYDROPHOBIC FILTER (7) is a single-use product and must be replaced each time it is used.
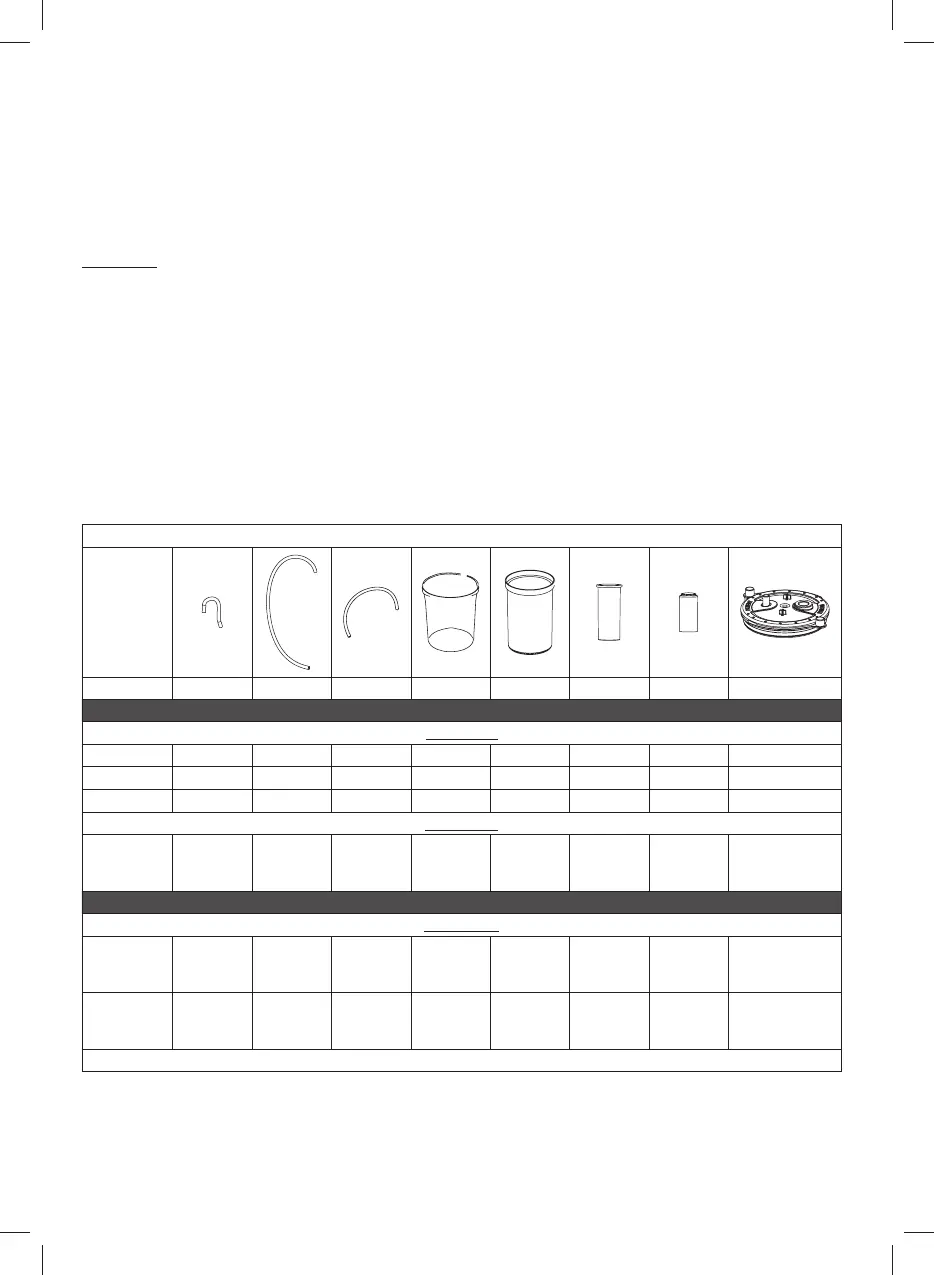
Table of planned methods / patient accessories
6a 6b 6c 8 9 10 10a 11
HYGIENIC PREPARATION IN THE HOME
Sanitisation
method A
✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓
method B
✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓
method C
✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓
Disinfection
✓
MAX 300
TIMES
✓
MAX 300
TIMES
✓
MAX 300
TIMES
\
✓
MAX 300
TIMES
✓
MAX 300
TIMES
✓
MAX 300
TIMES
✓
MAX 300
TIMES
HYGIENIC PREPARATION IN A CLINIC OR HOSPITAL
Sterilisation
method A
✓
MAX 50
TIMES
✓
MAX 50
TIMES
✓
MAX 50
TIMES
\ \ \ \ \
method B
\ \ \ \
✓
MAX 50
TIMES
✓
MAX 50
TIMES
✓
MAX 50
TIMES
✓
MAX 50
TIMES
✓: planned \: not planned
 Loading...
Loading...