Site Preparations
3-8 Ultrasound System – Common Service Information
Direction 5444964-100 English
Rev. 5
Electrical requirements for Console Ultrasound Systems (continued)
The following power line parameters should be monitored for
one week before installation. We recommend that you use an
analyzer Dranetz Model 606-3 or Dranetz Model 626:
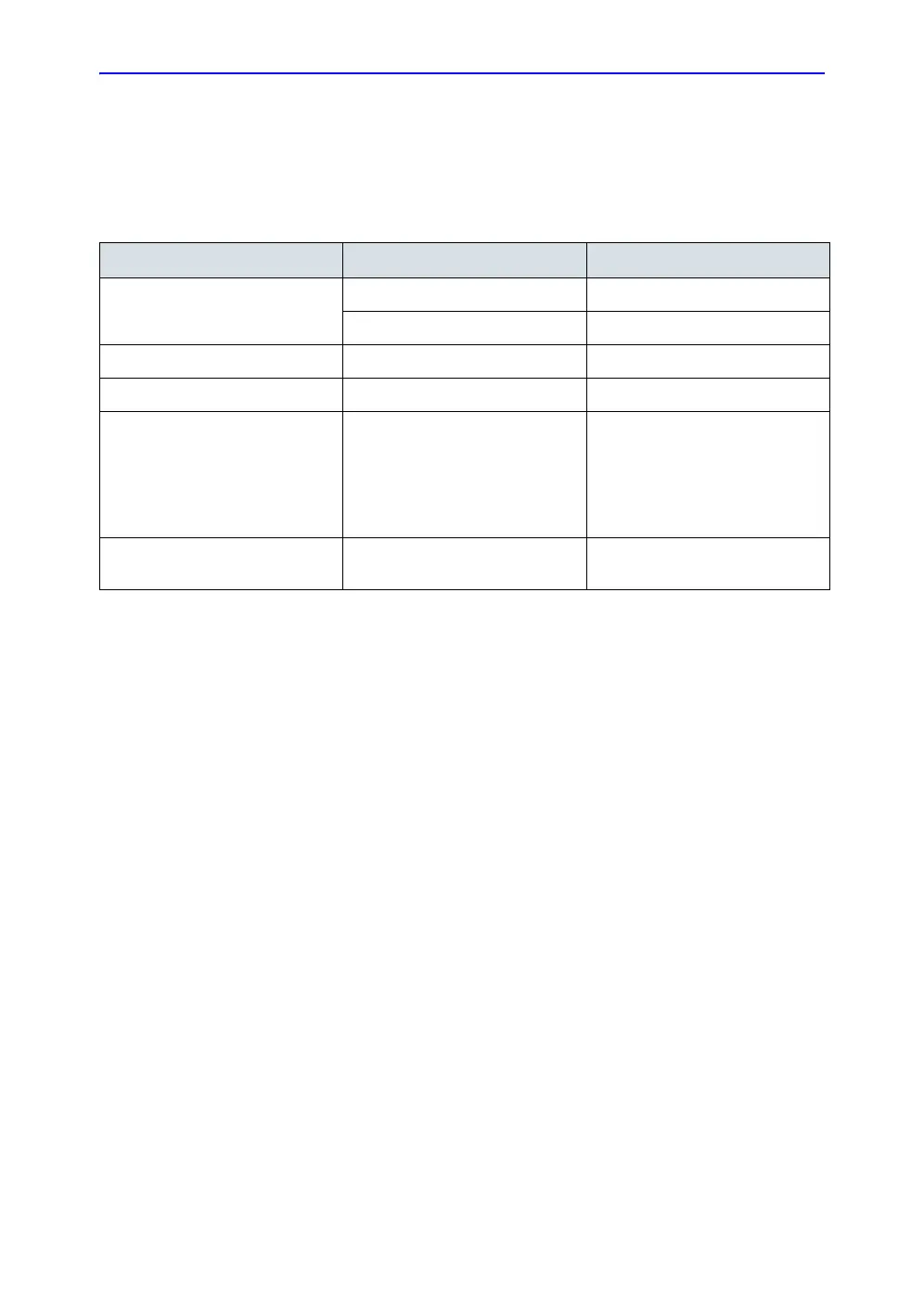
Table 3-4: Electrical Specifications for LOGIQ C Series and LOGIQ C3/C5 Premium
Parameter Area Limits
Voltage Range 100-120V~ 500VA
220-240V~ 500VA
Power All applications MAX. 750 VA
Line Frequency All applications 50/60Hz (±2Hz)
Power Transients All applications Less than 25% of nominal peak
voltage for less than 1 millisecond
for any type of transient, including
line frequency, synchronous,
asynchronous, or aperiodic
transients.
Decaying Oscillation All applications Less than 15% of peak voltage
for less than 1 millisecond.

 Loading...
Loading...