as an event with symptoms consistent with hypoglycemia requiring the assistance of another person
and associated with either a blood glucose below 50 mg/dL (≤56 mg/dL in the 5-year trial) or prompt
recovery after oral carbohydrate, intravenous glucose or glucagon administration.
The rates of severe symptomatic hypoglycemia in the LANTUS clinical trials (see Section 14 for a
description of the study designs) were comparable for all treatment regimens (see Tables 5 and 6). In
the pediatric phase 3 clinical trial, children and adolescents with type 1 diabetes had a higher incidence
of severe symptomatic hypoglycemia in the two treatment groups compared to the adult trials with type
1 diabetes. (see Table 5)
[See Clinical Studies (14)].
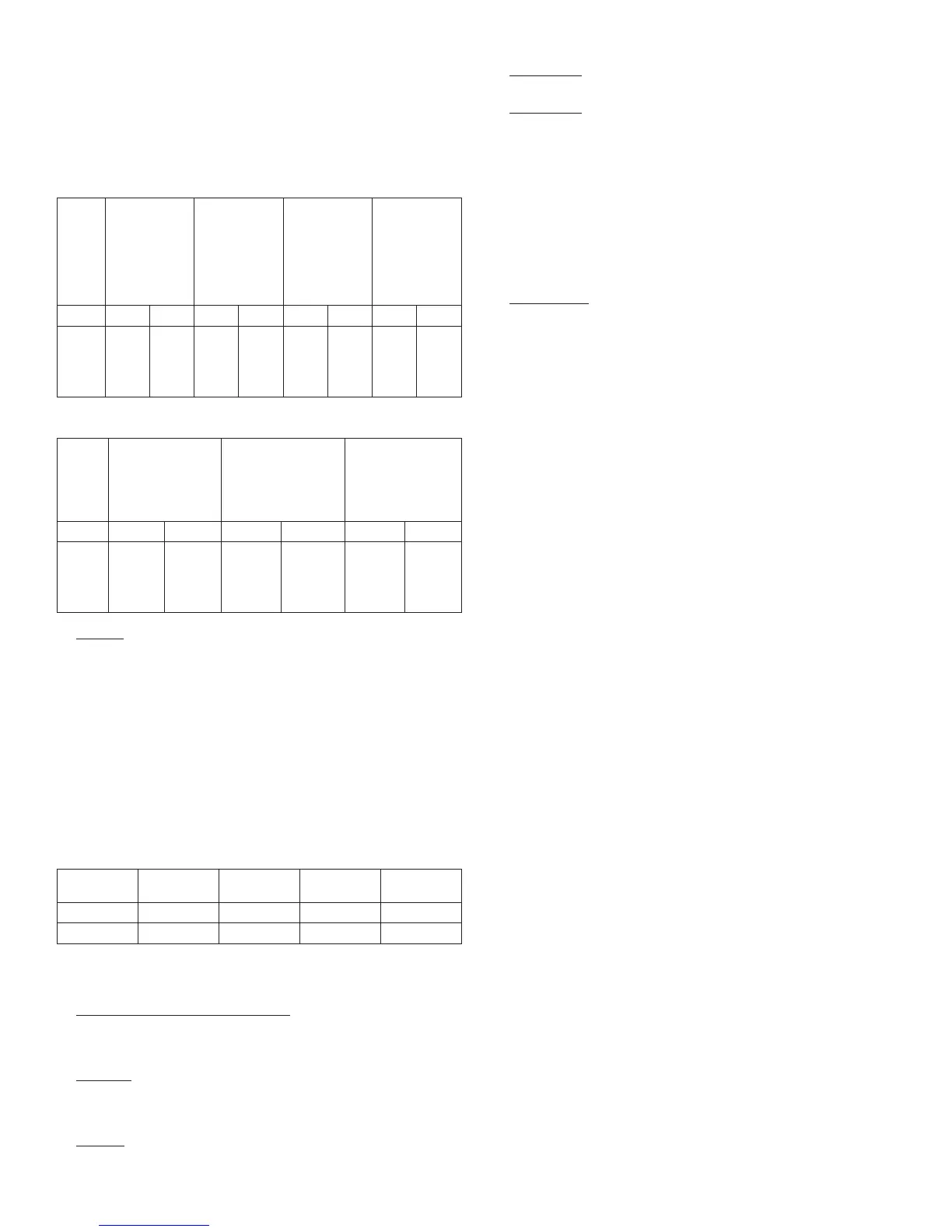
Table 5: Severe Symptomatic Hypoglycemia in Patients with Type 1 Diabetes
Study A
Type 1
Diabetes Adults
28 weeks
In combination
with regular
insulin
Study B
Type 1
Diabetes Adults
28 weeks
In combination
with regular
insulin
Study C
Type 1
Diabetes Adults
16 weeks
In combination
with insulin
lispro
Study D
Type 1
Diabetes
Pediatrics
26 weeks
In combination
with regular
insulin
LANTUS NPH LANTUS NPH LANTUS NPH LANTUS NPH
Percent
of
patients
(n/total
N)
10.6
(31/
292)
15.0
(44/
293)
8.7
(23/
264)
10.4
(28/
270)
6.5
(20/
310)
5.2
(16/
309)
23.0
(40/
174)
28.6
(50/
175)
Table 6: Severe Symptomatic Hypoglycemia in Patients with Type 2 Diabetes
Study E
Type 2
Diabetes Adults
52 weeks
In combination with
oral agents
Study F
Type 2
Diabetes Adults
28 weeks
In combination with
regular insulin
Study G
Type 2
Diabetes Adults
5 years
In combination with
regular insulin
LANTUS NPH LANTUS NPH LANTUS NPH
Percent
of
patients
(n/total
N)
1.7
(5/289)
1.1
(3/281)
0.4
(1/259)
2.3
(6/259)
7.8
(40/513)
11.9
(60/504)
•
Retinopathy
Retinopathy was evaluated in the LANTUS clinical studies by analysis of reported retinal adverse
events and fundus photography. The numbers of retinal adverse events reported for LANTUS and NPH
insulin treatment groups were similar for patients with type 1 and type 2 diabetes.
LANTUS was compared to NPH insulin in a 5-year randomized clinical trial that evaluated the
progression of retinopathy as assessed with fundus photography using a grading protocol derived from
the Early Treatment Diabetic Retinopathy Scale (ETDRS). Patients had type 2 diabetes (mean age 55
yrs) with no (86%) or mild (14%) retinopathy at baseline. Mean baseline HbA1c was 8.4%. The primary
outcome was progression by 3 or more steps on the ETDRS scale at study endpoint. Patients with
pre-specified post-baseline eye procedures (pan-retinal photocoagulation for proliferative or severe
nonproliferative diabetic retinopathy, local photocoagulation for new vessels, and vitrectomy for diabetic
retinopathy) were also considered as 3-step progressors regardless of actual change in ETDRS score
from baseline. Retinopathy graders were blinded to treatment group assignment. The results for the
primary endpoint are shown in Table 7 for both the per-protocol and Intent-to-Treat populations, and
indicate similarity of Lantus to NPH in the progression of diabetic retinopathy as assessed by this
outcome.
Table 7. Number (%) of patients with 3 or more step progression on ETDRS scale at
endpoint
Lantus (%) NPH (%) Difference
*,†
(SE)
95% CI for
difference
Per-protocol 53/374 (14.2%) 57/363 (15.7%) -2.0% (2.6%) -7.0% to +3.1%
Intent-to-Treat 63/502 (12.5%) 71/487 (14.6%) - 2.1% (2.1%) -6.3% to +2.1%
*Difference = Lantus – NPH
†using a generalized linear model (SAS GENMOD) with treatment and baseline HbA1c strata (cutoff
9.0%) as the classified independent variables, and with binomial distribution and identity link function
• Insulin initiation and intensification of glucose control
Intensification or rapid improvement in glucose control has been associated with a transitory, reversible
ophthalmologic refraction disorder, worsening of diabetic retinopathy, and acute painful peripheral
neuropathy. However, long-term glycemic control decreases the risk of diabetic retinopathy and
neuropathy.
•
Lipodystrophy
Long-term use of insulin, including LANTUS, can cause lipodystrophy at the site of repeated insulin
injections. Lipodystrophy includes lipohypertrophy (thickening of adipose tissue) and lipoatrophy
(thinning of adipose tissue), and may affect insulin absorption. Rotate insulin injection or infusion sites
within the same region to reduce the risk of lipodystrophy.
[See Dosage and Administration (2.1)].
• Weight gain
Weight gain can occur with insulin therapy, including LANTUS, and has been attributed to the anabolic
effects of insulin and the decrease in glucosuria.
• Peripheral Edema
Insulin, including LANTUS, may cause sodium retention and edema, particularly if previously poor
metabolic control is improved by intensified insulin therapy.
•
Allergic Reactions
Local Allergy
As with any insulin therapy, patients taking LANTUS may experience injection site reactions, including
redness, pain, itching, urticaria, edema, and inflammation. In clinical studies in adult patients, there was
a higher incidence of treatment-emergent injection site pain in LANTUS-treated patients (2.7%)
compared to NPH insulin-treated patients (0.7%). The reports of pain at the injection site did not result
in discontinuation of therapy.
Rotation of the injection site within a given area from one injection to the next may help to reduce or
prevent these reactions. In some instances, these reactions may be related to factors other than insulin,
such as irritants in a skin cleansing agent or poor injection technique. Most minor reactions to insulin
usually resolve in a few days to a few weeks.
Systemic Allergy
Severe, life-threatening, generalized allergy, including anaphylaxis, generalized skin reactions, an-
gioedema, bronchospasm, hypotension, and shock may occur with any insulin, including LANTUS and
may be life threatening.
•
Antibody production
All insulin products can elicit the formation of insulin antibodies. The presence of such insulin antibodies
may increase or decrease the efficacy of insulin and may require adjustment of the insulin dose. In
phase 3 clinical trials of LANTUS, increases in titers of antibodies to insulin were observed in NPH
insulin and insulin glargine treatment groups with similar incidences.
6.2 Postmarketing experience
The following adverse reactions have been identified during post-approval use of LANTUS.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always
possible to estimate reliably their frequency or establish a causal relationship to drug exposure.
Medication errors have been reported in which other insulins, particularly short-acting insulins, have
been accidentally administered instead of LANTUS
[See Patient Counseling Information (17)]. To avoid
medication errors between LANTUS and other insulins, patients should be instructed to always verify
the insulin label before each injection.
7. DRUG INTERACTIONS
A number of drugs affect glucose metabolism and may require insulin dose adjustment and particularly
close monitoring.
The following are examples of drugs that may increase the blood-glucose-lowering effect of insulins
including LANTUS and, therefore, increase the susceptibility to hypoglycemia: oral anti-diabetic
products, pramlintide, angiotensin converting enzyme (ACE) inhibitors, disopyramide, fibrates, fluox-
etine, monoamine oxidase inhibitors, propoxyphene, pentoxifylline, salicylates, somatostatin analogs,
and sulfonamide antibiotics.
The following are examples of drugs that may reduce the blood-glucose-lowering effect of insulins
including LANTUS: corticosteroids, niacin, danazol, diuretics, sympathomimetic agents (e.g., epineph-
rine, albuterol, terbutaline), glucagon, isoniazid, phenothiazine derivatives, somatropin, thyroid hor-
mones, estrogens, progestogens (e.g., in oral contraceptives), protease inhibitors and atypical
antipsychotic medications (e.g. olanzapine and clozapine).
Beta-blockers, clonidine, lithium salts, and alcohol may either potentiate or weaken the blood-glucose-
lowering effect of insulin. Pentamidine may cause hypoglycemia, which may sometimes be followed
by hyperglycemia.
The signs of hypoglycemia may be reduced or absent in patients taking sympatholytic drugs such as
beta-blockers, clonidine, guanethidine, and reserpine.
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: Subcutaneous reproduction and teratology studies have been performed with
insulin glargine and regular human insulin in rats and Himalayan rabbits. Insulin glargine was given to
female rats before mating, during mating, and throughout pregnancy at doses up to 0.36 mg/kg/day,
which is approximately 7 times the recommended human subcutaneous starting dose of 10 Units/day
(0.008 mg/kg/day), based on mg/m
2
. In rabbits, doses of 0.072 mg/kg/day, which is approximately 2
times the recommended human subcutaneous starting dose of 10 Units/day (0.008 mg/kg/day), based
on mg/m
2
, were administered during organogenesis. The effects of insulin glargine did not generally
differ from those observed with regular human insulin in rats or rabbits. However, in rabbits, five fetuses
from two litters of the high-dose group exhibited dilation of the cerebral ventricles. Fertility and early
embryonic development appeared normal.
There are no well-controlled clinical studies of the use of LANTUS in pregnant women. Because animal
reproduction studies are not always predictive of human response, this drug should be used during
pregnancy only if the potential benefit justifies the potential risk to the fetus. It is essential for patients
with diabetes or a history of gestational diabetes to maintain good metabolic control before conception
and throughout pregnancy. Insulin requirements may decrease during the first trimester, generally
increase during the second and third trimesters, and rapidly decline after delivery. Careful monitoring
of glucose control is essential in these patients.
8.3 Nursing Mothers
It is unknown whether insulin glargine is excreted in human milk. Because many drugs, including human
insulin, are excreted in human milk, caution should be exercised when LANTUS is administered to a
nursing woman. Use of LANTUS is compatible with breastfeeding, but women with diabetes who are
lactating may require adjustments of their insulin doses.
8.4 Pediatric Use
The safety and effectiveness of subcutaneous injections of LANTUS have been established in pediatric
patients (age 6 to 15 years) with type 1 diabetes
[see Clinical Studies (14)]. LANTUS has not been
studied in pediatric patients younger than 6 years of age with type 1 diabetes. LANTUS has not been
studied in pediatric patients with type 2 diabetes.
Based on the results of a study in pediatric patients, the dose recommendation when switching to
LANTUS is the same as that described for adults
[see Dosage and Administration (2.3) and Clinical
Studies (14)].
As in adults, the dosage of LANTUS must be individualized in pediatric patients based
on metabolic needs and frequent monitoring of blood glucose.
8.5 Geriatric Use
In controlled clinical studies comparing LANTUS to NPH insulin, 593 of 3890 patients (15%) with type
1 and type 2 diabetes were ≥65 years of age and 80 (2%) patients were ≥75 years of age. The only
difference in safety or effectiveness in the subpopulation of patients ≥65 years of age compared to the
entire study population was a higher incidence of cardiovascular events typically seen in an older
population in both LANTUS and NPH insulin-treated patients.
3

 Loading...
Loading...