Hypoglycemia can be mild to severe. Its onset may be rapid. Some patients have few or no warning
symptoms, including:
• patients with diabetes for a long time
• patients with diabetic neuropathy (nerve problems)
• or patients using certain medicines for high blood pressure or heart problems.
Hypoglycemia may reduce your ability to drive a car or use mechanical equipment and you may risk
injury to yourself or others.
Severe hypoglycemia can be dangerous and can cause temporary or permanent harm to your heart
or brain. It may cause unconsciousness, seizures, or death.
Symptoms of hypoglycemia may include:
• anxiety, irritability, restlessness, trouble concentrating, personality changes, mood changes, or
other abnormal behavior
• tingling in your hands, feet, lips, or tongue
• dizziness, light-headedness, or drowsiness
• nightmares or trouble sleeping
• headache
• blurred vision
• slurred speech
• palpitations (fast heart beat)
• sweating
• tremor (shaking)
• unsteady gait (walking).
If you have hypoglycemia often or it is hard for you to know if you have the symptoms of hypoglycemia,
talk to your healthcare provider.
Mild to moderate hypoglycemia is treated by eating or drinking carbohydrates such as fruit juice, raisins,
sugar candies, milk or glucose tablets. Talk to your healthcare provider about the amount of
carbohydrates you should eat to treat mild to moderate hypoglycemia.
Severe hypoglycemia may require the help of another person or emergency medical people. A person
with hypoglycemia who is unable to take foods or liquids with sugar by mouth, or is unconscious needs
medical help fast and will need treatment with a glucagon injection or glucose given intravenously (IV).
Without medical help right away, serious reactions or even death could happen.
Hyperglycemia (high blood sugar):
Hyperglycemia happens when you have too much sugar in your blood. Usually, it means there is not
enough insulin to break down the food you eat into energy your body can use. Hyperglycemia can be
caused by a fever, an infection, stress, eating more than you should, taking less insulin than prescribed,
or it can mean your diabetes is getting worse.
Hyperglycemia can happen with:
• Insufficient (too little) insulin. This can happen from:
- injecting too little or no insulin
- incorrect storage (freezing, excessive heat)
- use after the expiration date.
• Too much carbohydrate intake. This can happen if you eat larger meals, eat more often, or
increase the amount of carbohydrate in your meals.
• Medicines that affect insulin. Be sure to discuss all your medicines with your healthcare provider.
Do not start any new medicines until you know how they may affect your insulin dose.
• Medical conditions that affect insulin. These medical conditions include fevers, infections, heart
attacks, and stress.
• Injecting insulin the wrong way or in the wrong injection area.
Testing your blood or urine often will let you know if you have hyperglycemia. If your tests are often
high, tell your healthcare provider so your dose of insulin can be changed.
Hyperglycemia can be mild or severe. It can progress to diabetic ketoacidosis (DKA) or very high
glucose levels (hyperosmolar coma) and result in unconsciousness and death.
Although diabetic ketoacidosis occurs most often in patients with type 1 diabetes, it can also happen
in patients with type 2 diabetes who become very sick. Because some patients get few symptoms of
hyperglycemia, it is important to check your blood sugar/urine sugar and ketones regularly.
Symptoms of hyperglycemia include:
• confusion or drowsiness
• increased thirst
• decreased appetite, nausea, or vomiting
• rapid heart rate
• increased urination and dehydration (too little fluid in your body).
Symptoms of DKA also include:
• fruity smelling breath
• fast, deep breathing
• stomach area (abdominal) pain.
Severe or continuing hyperglycemia or DKA needs evaluation and treatment right away by your
healthcare provider.
Do not use LANTUS to treat diabetic ketoacidosis.
Other possible side effects of LANTUS include:
Serious allergic reactions:
Some times severe, life-threatening allergic reactions can happen with insulin. If you think you are
having a severe allergic reaction, get medical help right away. Signs of insulin allergy include:
• rash all over your body
• shortness of breath
• wheezing (trouble breathing)
• fast pulse
• sweating
• low blood pressure.
Reactions at the injection site:
Injecting insulin can cause the following reactions on the skin at the injection site:
• little depression in the skin (lipoatrophy)
• skin thickening (lipohypertrophy)
• red, swelling, itchy skin (injection site reaction).
You can reduce the chance of getting an injection site reaction if you change (rotate) the injection site
each time. An injection site reaction should clear up in a few days or a few weeks. If injection site
reactions do not go away or keep happening call your healthcare provider.
Tell your healthcare provider if you have any side effects that bother you.
These are not all the side effects of LANTUS. Ask your healthcare provider or pharmacist for more
information.
How should I store LANTUS?
• Unopened cartridge system:
Store new unopened LANTUS cartridge systems in a refrigerator (not the freezer) between 36 °F
to 46 °F (2°C to 8°C). Do not freeze LANTUS. Keep LANTUS out of direct heat and light. If a
cartridge system has been frozen or overheated, throw it away.
• Open (In-Use) cartridge system:
Once a cartridge system is opened, you can keep it at room temperature (below 86°F [30°C]) but
away from direct heat and light for 28 days. Cartridge system in OptiClik
®
insulin Pen must be
discarded 28 days after the first use even if it still contains LANTUS. The opened cartridge system
in OptiClik
®
insulin Pen should be kept at room temperature (below 86°F [30°C]) and away from
direct heat and light for up to 28 days. For example, do not leave it in a car on a summer day.
Do not store OptiClik
®
, with or without cartridge system, in a refrigerator at any time.
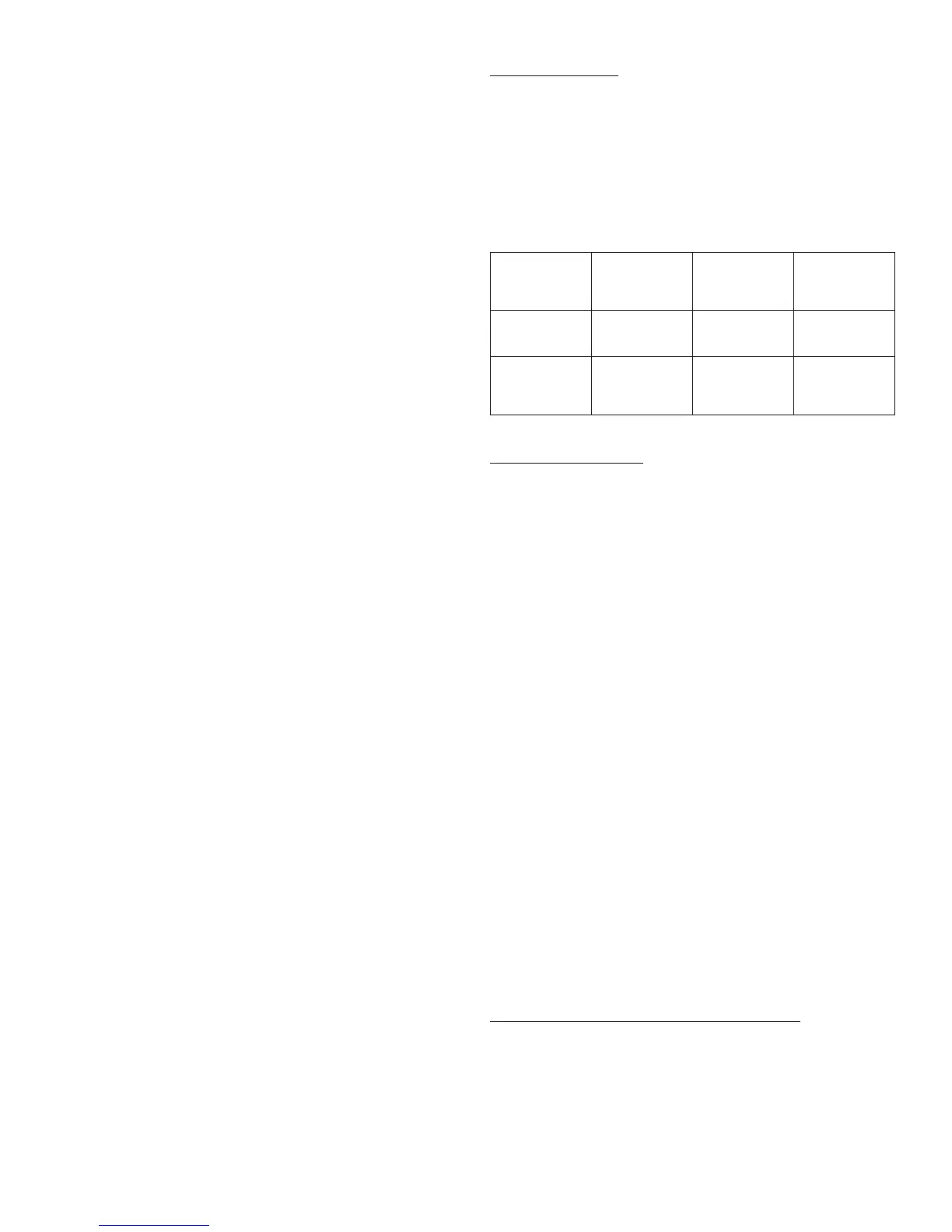
These storage conditions are summarized in the following table:
Not in-use
(unopened)
Refrigerated
Not in-use
(unopened)
Room Temperature
In-use
(opened)
(See Temperature
Below)
3 mL Cartridge
System
Until expiration date 28 days
28 days
Refrigerated or room
temperature
3 mL cartridge
system inserted in
OptiClik
®
insulin Pen
28 days
Room temperature
only
(Do not refrigerate)
• Do not use a cartridge system of LANTUS after the expiration date stamped on the label.
• Do not use LANTUS if it is cloudy, colored, or if you see particles.
General Information about LANTUS
• Use LANTUS only to treat your diabetes. Do not give or share LANTUS with another person, even
if they have diabetes also. It may harm them.
• This leaflet summarizes the most important information about LANTUS. If you would like more
information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist
for information about LANTUS that is written for healthcare professionals. For more information
about LANTUS call 1-800-633-1610 or go to website www.lantus.com.
ADDITIONAL INFORMATION
DIABETES FORECAST is a national magazine designed especially for patients with diabetes and their
families and is available by subscription from the American Diabetes Association (ADA), P.O. Box 363,
Mt. Morris, IL 61054-0363, 1-800-DIABETES (1-800-342-2383). You may also visit the ADA website at
www.diabetes.org.
Another publication, COUNTDOWN, is available from the Juvenile Diabetes Research Foundation
International (JDRF), 120 Wall Street, 19th Floor, New York, New York 10005, 1-800-JDF-CURE
(1-800-533-2873). You may also visit the JDRF website at www.jdf.org.
To get more information about diabetes, check with your healthcare professional or diabetes educator
or visit www.DiabetesWatch.com.
Additional information about LANTUS can be obtained by calling 1-800-633-1610 or by visiting
www.lantus.com.
Rev. March 2007
sanofi-aventis U.S. LLC
Bridgewater, NJ 08807
©2007 sanofi-aventis U.S. LLC
OptiClik
®
is a registered trademark of sanofi-aventis U.S. LLC
†
The brands listed are the trademarks of their respective owners and are not trademarks of
sanofi-aventis U.S. LLC
Patient Information
LANTUS
®
SOLOSTAR
®
3 mL disposable insulin delivery device (300 units per device)
100 units per mL (U-100)
(insulin glargine [recombinant DNA origin] injection)
• What is the most important information I should know about LANTUS?
• What is LANTUS?
• Who should NOT take LANTUS?
• How should I use LANTUS?
• Mixing with LANTUS
• Instructions for Use
• What can affect how much insulin I need?
• What are the possible side effects of LANTUS and other insulins?
• How should I store LANTUS?
• General Information about LANTUS
Read this ″Patient Information″ that comes with LANTUS (LAN-tus) before you start using it and each
time you get a refill because there may be new information. This leaflet does not take the place of
talking with your healthcare provider about your condition or treatment. If you have questions about
LANTUS or about diabetes, talk with your healthcare provider.
What is the most important information I should know about LANTUS?
• Do not change the insulin you are using without talking to your healthcare provider. Any
change of insulin should be made cautiously and only under medical supervision. Changes in
insulin strength, manufacturer, type (for example: Regular, NPH, analogs), species (beef, pork,
beef-pork, human) or method of manufacture (recombinant DNA versus animal-source insulin)
may need a change in the dose. This dose change may be needed right away or later on during
the first several weeks or months on the new insulin. Doses of oral anti-diabetic medicines may
also need to change, if your insulin is changed.
• You must test your blood sugar levels while using an insulin, such as LANTUS. Your
healthcare provider will tell you how often you should test your blood sugar level, and what to do
if it is high or low.
• Do NOT dilute or mix LANTUS with any other insulin or solution. It will not work and you may
lose blood sugar control, which could be serious.
10
 Loading...
Loading...