Although diabetic ketoacidosis occurs most often in patients with type 1 diabetes,it can also happen
in patients with type 2 diabetes who become very sick. Because some patients get few symptoms of
hyperglycemia, it is important to check your blood sugar/urine sugar and ketones regularly.
Symptoms of hyperglycemia include:
• confusion or drowsiness
• increased thirst
• decreased appetite, nausea, or vomiting
• rapid heart rate
• increased urination and dehydration (too little fluid in your body).
Symptoms of DKA also include:
• fruity smelling breath
• fast, deep breathing
• stomach area (abdominal) pain.
Severe or continuing hyperglycemia or DKA needs evaluation and treatment right away by your
healthcare provider.
Do not use LANTUS to treat diabetic ketoacidosis.
Other possible side effects of LANTUS include:
Serious allergic reactions:
Some times severe, life-threatening allergic reactions can happen with insulin. If you think you are
having a severe allergic reaction, get medical help right away. Signs of insulin allergy include:
• rash all over your body
• shortness of breath
• wheezing (trouble breathing)
• fast pulse
• sweating
• low blood pressure.
Reactions at the injection site:
Injecting insulin can cause the following reactions on the skin at the injection site:
• little depression in the skin (lipoatrophy)
• skin thickening (lipohypertrophy)
• red, swelling, itchy skin (injection site reaction).
You can reduce the chance of getting an injection site reaction if you change (rotate) the injection site
each time. An injection site reaction should clear up in a few days or a few weeks. If injection site
reactions do not go away or keep happening call your healthcare provider.
Tell your healthcare provider if you have any side effects that bother you.
These are not all the side effects of LANTUS. Ask your healthcare provider or pharmacist for more
information.
How should I store LANTUS?
• Unopened SoloStar
®
:
Store new unopened SoloStar
®
disposable insulin pen in a refrigerator (not the freezer) between
36°F to 46°F (2°C to 8°C). Do not freeze LANTUS. Keep LANTUS out of direct heat and light.
If a diposable insulin pen has been frozen or overheated, throw it away.
• Open (In-Use) SoloStar
®
:
Once SoloStar
®
is opened (in-use), SoloStar
®
should NOT be refrigerated but should be kept at
room temperature (below 86°F [30°C]) away from direct heat and light. The opened (in-use)
SoloStar
®
kept at room temperature must be discarded after 28 days.
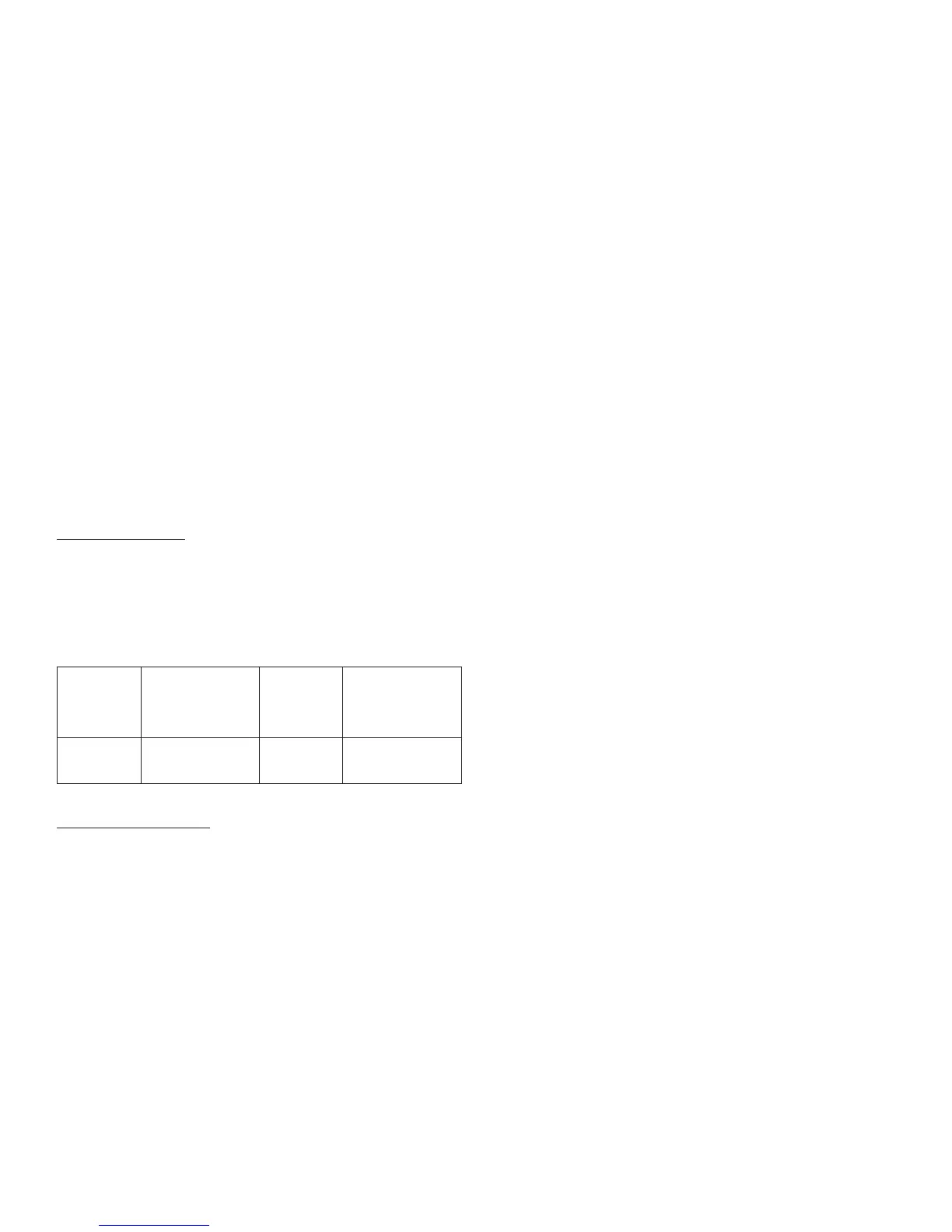
These storage conditions are summarized in the following table:
Not in-use
(unopened)
Refrigerated
Not in-use
(unopened)
Room
Temperature
In-use
(opened)
Room Temperature
(Do not refrigerate)
3 mL SoloStar
®
dispoable insulin
device
Until expiration date 28 days 28 days
• Do not use SoloStar
®
with LANTUS after the expiration date stamped on the label.
• Do not use LANTUS if it is cloudy, colored, or if you see particles.
General Information about LANTUS
• Use LANTUS only to treat your diabetes. Do not give or share LANTUS with another person, even
if they have diabetes also. It may harm them.
• This leaflet summarizes the most important information about LANTUS. If you would like more
information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist
for information about LANTUS that is written for healthcare professionals. For more information
about LANTUS call 1-800-633-1610 or go to website www.lantus.com.
ADDITIONAL INFORMATION
DIABETES FORECAST is a national magazine designed especially for patients with diabetes and their
families and is available by subscription from the American Diabetes Association (ADA), P.O. Box 363,
Mt. Morris, IL 61054-0363, 1-800-DIABETES (1-800-342-2383). You may also visit the ADA website at
www.diabetes.org.
Another publication, COUNTDOWN, is available from the Juvenile Diabetes Research Foundation
International (JDRF), 120 Wall Street, 19th Floor, New York, New York 10005, 1-800-JDF-CURE
(1-800-533-2873). You may also visit the JDRF website at www.jdf.org.
To get more information about diabetes, check with your healthcare professional or diabetes educator
or visit www.DiabetesWatch.com.
Additional information about LANTUS can be obtained by calling 1-800-633-1610 or by visiting
www.lantus.com.
Rev. March 2007
sanofi-aventis U.S. LLC
Bridgewater, NJ 08807
©2007 sanofi-aventis U.S. LLC
Lantus
®
and SoloStar
®
are a registered trademark of sanofi-aventis U.S. LLC
†
The brands listed are the trademarks of their respective owners and are not trademarks of
sanofi-aventis U.S. LLC
GLA-FPLR-SL-APR10 Rx Only
12
 Loading...
Loading...