INSTRUCTIONS FOR USE
RETROGRADE INSERTION
INDICATIONS FOR USE:
• The Medcomp
®
Split-Stream
®
is indicated for use in attaining
Long-Term vascular access for Hemodialysis and Apheresis.
• It may be inserted percutaneously and is primarily placed in the
internal jugular vein.
• Alternate insertion sites include the subclavian vein.
• Catheters greater than 40cm are intended for femoral vein insertion.
CONTRAINDICATIONS:
• This catheter is intended for Long-Term vascular access only and
should NOT be used for any purpose other than indicated in these
instructions.
• To maintain peak performance of the Split-Stream
®
extension set, it
is recommended that the extension set be replaced every 6 months.
WARNINGS:
• In the rare event that a hub or connector separates from any
component during insertion or use, take all necessary steps and
precautions to prevent blood loss or air embolism and remove
catheter.
• Do not advance the guidewire or catheter if unusual resistance is
encountered.
• Do not insert or withdraw the guidewire forcibly from any
component. The wire may break or unravel. If the guidewire
becomes damaged, the introducer needle or Vascu-Sheath
®
introducer and guidewire must be removed together.
• Federal Law (USA) restricts this device to sale by or on the order of
a physician.
• This catheter is for Single Use Only.
• Re-use may lead to infection or illness/injury.
• Do not resterilize the catheter or accessories by any method.
• The manufacturer shall not be liable for any damages caused by re-
use or resterilization of this catheter or accessories.
• Contents sterile and non-pyrogenic in unopened, undamaged
package. STERILIZED BY ETHYLENE OXIDE
• Do not use catheter or accessories if package is opened or damaged.
• Do not use catheter or accessories if any sign of product damage is
visible.
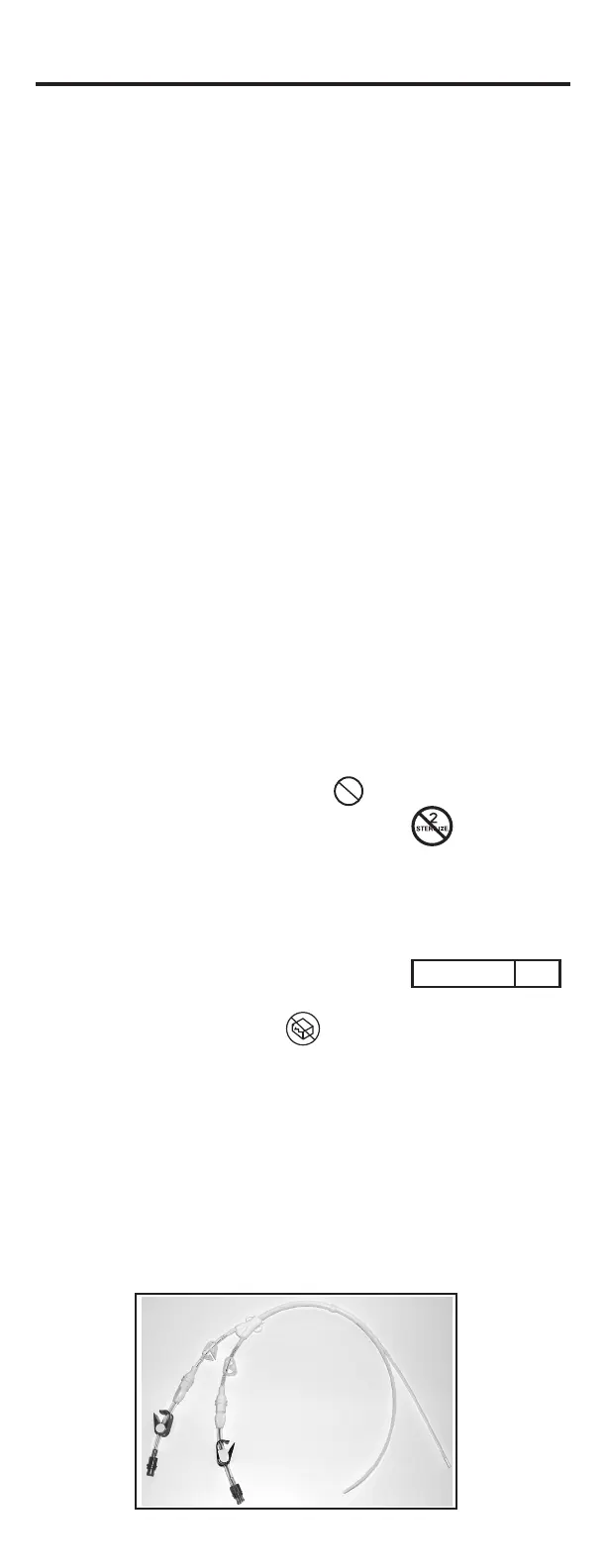
DESCRIPTION:
• The versatility of the Split-Stream
®
allows the lumens to be split to
form two free oating lumens to help eliminate catheter occlusion by
the vessel.
• The Split-Stream
®
is manufactured from soft radiopaque
polyurethane material which provides increased patient comfort
while providing excellent biocompatibility.
-11-
2
STERILE EO
 Loading...
Loading...