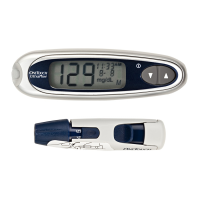
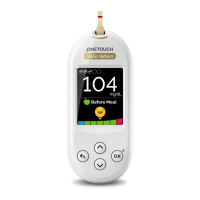
AW 06985406A
© 2018–2020 LifeScan
IP Holdings, LLC
Contents covered by one or more of the following U.S. patents: 6,733,655,
7,462,265, 7,468,125, 8,066,866, 8,093,903, 6,602,191, 6,976,958, and 7,156,809.
Purchase of this device does not act to grant a use licence under these patents.
Such a licence is granted only when the device is used with OneTouchSelect
®
Plus
Test Strip. No test strip supplier other than LifeScan is authorised to grant such a
licence. The accuracy of results generated with LifeScan meters using test strips
manufactured by anyone other than LifeScan has not been evaluated by LifeScan.
LifeScan self-test blood glucose
monitoring devices conform to the
following EU Directives:
IVDD (98/79/EC):
Blood Glucose Meter,
Test Strips, and Control
Solution
MDD (93/42/EEC):
Lancets
Lancing Device
RED (2014/53/EU):
Rev. Date: 02/2020
Blood Glucose
Meter
LifeScan Europe GmbH
Gubelstrasse 34
6300 Zug
Switzerland
Contact LifeScan
Customer Service at:
service@LifeScanMEA.com.
TRA
REGISTERED No:
ER41149/15
DEALER No:
DA45564/15
Jordan Telecommunications
Approval
TRC/LPD/2015/112
Meter Made in China
023-413
 Loading...
Loading...