Do you have a question about the Zimmer Sono One and is the answer not in the manual?
Symbol indicating a hazardous situation, requires caution.
Symbol indicating potential device damage, requires caution.
Identifies the electrical safety classification of the applied part.
Indicates the need to adhere to the user manual.
Unique identifier for the specific device unit.
Code number for the product model.
Information about the company that produced the device.
Indicates when the device was produced.
Device meets Class II safety standards.
Device heats tissue for musculoskeletal system disorders.
Conditions and diseases for which the device is intended.
Conditions or situations where the device should not be used.
Notes on using the device with patients having implants.
Measures to prevent electric shock during maintenance.
Ensuring proper earthing and using supplied cables.
Keeping device away from strong electromagnetic sources.
Device is not suitable for explosive or flammable areas.
Ensuring accessibility for disconnection and pre-use checks.
Preventing damage to the ultrasound head from sharp objects.
Position device for easy access to power for disconnection.
Protect the transducer from sharp or pointed objects.
Only use the specified SonoPlus ultrasound gel.
Only use accessories provided by Zimmer MedizinSysteme.
Manage potential device interference with nearby equipment.
Do not alter the device or medical system components.
Protective measures for users when the transducer is in water.
Device is intended solely for trained medical personnel.
Improper use can lead to hazardous energy release.
Patients must not be left unattended during treatment.
Strict adherence to instructions from medical experts is required.
Device must not be operated in damp or wet conditions.
Dispose of packaging properly, keeping it away from children.
Assess risks before treating patients with implants or electronic devices.
Do not connect the patient to HF surgical equipment simultaneously.
Shows available therapy programs for selection.
Displays active treatment parameters and status.
Interface for device settings and adjustments.
Visualizes current therapy parameter settings.
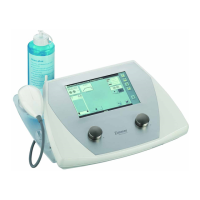
Description of buttons, sockets, and the ultrasound head.
Explanation of screens, displays, windows, and buttons.
Adjusting intensity, starting/stopping, and setting time/frequency.
Information shown on time and coupling displays.
How to return to the programme screen.
Details on how intensity is displayed (W, W/cm²).
Graphical representation of the set intensity level.
Setting the threshold for acceptable coupling.
Choosing the display units for intensity.
Information about the device's software version.
How to stop current processes and return to previous screens.
Procedure to save settings and exit configuration.
Entry point for technical maintenance functions.
Adjusting operating mode, frequency, and duty cycle.
Description of the main power switch for the unit.
Socket for connecting the power supply cable.
Port for attaching the ultrasound transducer.
Details and specifications of the 5 cm² ultrasound head.
Indicates the quality of the ultrasound coupling.
Component for storing the ultrasound transducer.
Steps to connect the device cables correctly.
Procedure for selecting a program and initiating treatment.
Electrical input requirements and power draw.
Device's protection class and transducer classification.
Device size, weight, and environmental operating limits.
Specifications for handling the device during storage/transit.
Frequency, intensity levels, and maximum power of the head.
Device precision and available ultrasound wave types.
Information on replacing the ultrasound transducer.
Essential safety steps before starting cleaning or maintenance.
Steps to take if liquid enters the device.
Ensuring inscriptions remain legible after cleaning.
The device is considered non-critical for hygiene on healthy skin.
Methods for cleaning the exterior surfaces with approved cleaners.
Guidelines for how often disinfection should occur.
Using approved disinfectants and wiping techniques.
Allowing flammable disinfectants to fully evaporate before use.
Ensuring the device is used in a clean and sanitized setting.
Confirmation of conformity with EC directives for medical devices.
Details of Zimmer MedizinSysteme GmbH.
Components included in the standard package.
Sono One is not for use with other medical devices.
List of system error codes and their interpretations.
Procedure to verify device operation and coupling performance.
Address for inquiries and equipment malfunction assistance.
Address for questions and technical support.
Adherence to specific German medical device regulations.
Guidelines for returning the device for disposal.
Following country-specific rules for waste disposal.
Special measures for ensuring EMC compliance.
Requirements for the electromagnetic environment regarding emissions.
Requirements for the electromagnetic environment regarding immunity.
Device performance under conducted RF interference.
Device performance under radiated RF interference.
Minimum safe distances from transmitters.
| Brand | Zimmer |
|---|---|
| Model | Sono One |
| Category | Medical Equipment |
| Language | English |