Product Information
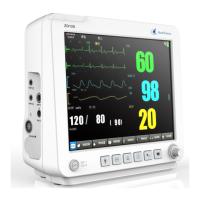
Product model: ZD120
Product name: Patient Monitor
Manufacturer
Shenzhen Hexin ZONDAN Medical Equipment Co., Ltd.
Registered/Production address:
Floor 14, Block D, Dianlian Technology Building,the Crossing between South Circle
Road and South Fuli Road,Guangming District, 518106 Shenzhen PEOPLE'S
REPUBLIC OF CHINA
Tel: +86 755 26865970 Fax: +86 755 26860497
Edition
Second Edition: October 2021
Version: V1.2
MEDHealth supplies (Pty) Ltd
All Rights Reserved.
Distributor
MEDHealth Supplies (Pty) Ltd
Corner of Barbara and North Reef Road
Elandsfontein, Henville, 1429, Germiston, Johannesburg, Gauteng, South Africa
Reg No.: 2021/385832/07 Telephone: +27 10 013 3010
E-mail: admin@medhealthsup.com
Regulatory and Safety Specifications
Standard
The product is made under the ISO13485 quality system certified by TUV PS. The
product has passed the CE certification.
Declaration
The ZD120 Patient Monitor is a Class IIa device and complies with the requirements of
the Council Directive 93/42/EEC concerning medical devices and carries CE-marking
accordingly.
Authorized EU Representative
Shanghai International Holding Corp.GmbH (Europe)
Eiffestrasse 80, 20537 Hamburg, Germany
Tel: 0049-40-2513175 Fax: 0049-40-255726