COPYRIGHT © 2019-2020, ACCELERATED CARE PLUS CORP., ALL RIGHTS RESERVED
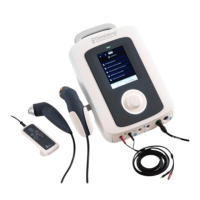
OmniSWD
®
Shortwave Diathermy System
User Manual
Issued 08.04.2020
Part No. 2903030M Rev 7
This manual and all content therein is owned exclusively by Accelerated Care Plus ("ACP") and is protected by copyright. This manual or any
portion thereof may not be photocopied, reproduced or translated to another language without the express prior written consent of ACP. This
manual may only be used by entities that have purchased the equipment or have implemented the ACP program and are covered by an executed
lease agreement. This manual may not be used for any other purpose.
Any additional copies of the Manual shall be ordered from ACP. No changes or modifications shall be made to the Manual without prior review
and written authorization from ACP. No authorization is given to market, sell, disclose, or exploit this Manual except as for purposes of using the
Equipment as contemplated by the Lease Agreement.
ACCELERATED CARE PLUS MAKES NO WARRANTY OF ANY KIND WITH REGARD TO THIS MANUAL, INCLUDING, BUT NOT
LIMITED TO, THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. Accelerated
Care Plus shall not be liable for errors contained herein or for incidental or consequential damages in connection with the furnishing, performance
or use of this Manual.
The information contained in this document is subject to change without notice.