4
Model overview
This booklet is valid for the following hearing aid
families and models:
Viron FW4
■ Viron 9 – VN9 B 105
GTIN: (01)05714464021253
■ Viron 7 – VN7 B 105
GTIN: (01)05714464021260
■ Viron 5 – VN5 B 105
GTIN: (01)05714464021277
■ Viron 3 – VN3 B 105
GTIN: (01)05714464021284
■ Viron 1 – VN1 B 105
GTIN: (01)05714464021291
5
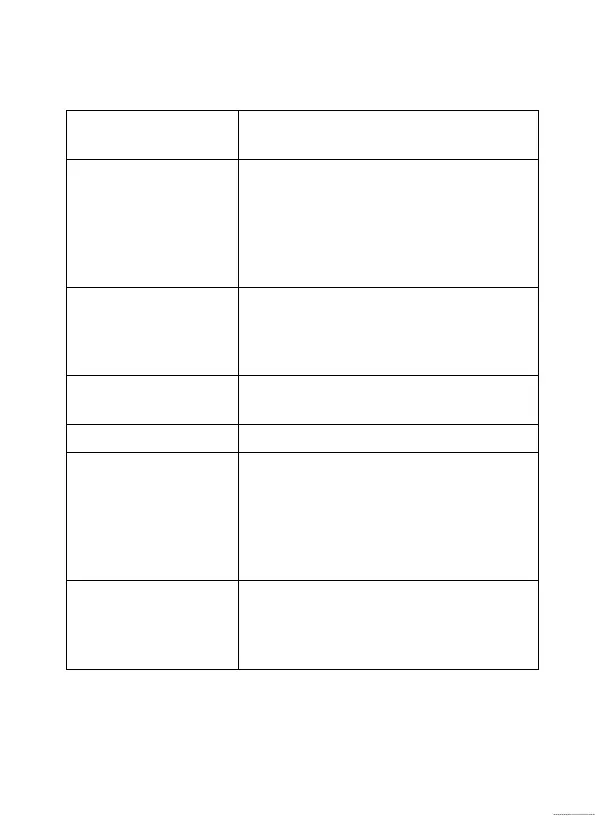
Intended use
Intended use The hearing aid is intended to amplify
and transmit sound to the ear .
Indications for use Bilateral or unilateral impaired
hearing of sensorineural, conductive,
or mixed type ranging from a slight
(16 dB HL*) to severe (85 dB HL*)
degree of hearing loss, with an
individual frequency confi guration.
Intended user Person with hearing loss using a
hearing aid and their caregivers.
Hearing care professional responsible
for adjusting the hearing aid.
Intended user
group
Adults and children older than
36 months.
Use environment Indoor and outdoor .
Contraindications Not suitable for infants below
36 months. Users of active implants
must pay special attention when
using the hearing aid. For more
information read the Warnings
section.
Clinical benefi ts The hearing aid is designed to
provide better speech understanding
to help ease communication with the
aim of improving quality of life.
* As specifi ed by the American Speech-Language-Hearing
Association, asha.org, using pure-tone average of 0.5, 1 and 2 kHz
 Loading...
Loading...











