Possible Problems and resolutions
Problems Possible causes Solution
There is no
response to
the function
button
The button can not be pressed to
its position
Ensure that the button is
fully depressed.
Battery capacities are low
The batteries may be
missing, discharged, or
oriented incorrectly.
Replaced them with new
ones.
The Pulse
search time is
too long
Perfusion may be too low
Check the patient. Change
the measuring site. Try
another oximeter.
Patient movement
Interference due to patient
activity may be preventing
the oximeter from tracking
the pulse. Keep the patient
still, if possible.
Electromagnetic interference
may be preventing the oximeter
from tracking the pulse.
Remove the source of
interference.
There may be interference due
to ambient light, or the oximeter
may be on an extremity with a
blood pressure cuff, arterial
catheter, or intravascular line.
Reposition oximeter, as
necessary.
Display is
dark-or-bright
Battery capacities are low. Replace the batteries.
Symbols Definitions
Symbol
Definition
Type BF equipment (Refer to IEC 60601-1)
%SpO
2
Oxygen saturation of arterial blood
/Min
Pulse rate
Non-Alarm indication (The device does not have alarm function)
IPX1
Enclosure degree of ingress protection.
Serial number
Refer to this user’s manual.
Symbol for the marking of electrical and electronics devices
according to Directive 2002/96/EC.
The device, accessories and the packaging have to be disposed of
waste correctly at the end of the usage. Please follow Local
Ordinances or Regulations for disposal.
Note: The Oximeter is applied to this regulation.
Guidance and manufacture’s declaration – electromagnetic
ermissions-for all EQUIPMENT and SYSTEMS
Guidance and manufacture’s declaration – electromagnetic emission
The Fingertip Pulse Oximeter is intended for use in the electromagnetic
environment specified below. The customer of the user of the Fingertip Pulse
Oximeter should assure that it is used in such and environment.
Emission
test
Compliance Electromagnetic environment – guidance
RF
emissions
CISPR 11
Group 1 The Fingertip Pulse Oximeter uses RF energy
only for its internal function. Therefore, its RF
emissions are very low and are not likely to
cause any interference in nearby electronic
equipment.
RF
emission
CISPR 11
Class B The Fingertip Pulse Oximeter is suitable for use
in all establishments, including domestic
establishments and those directly connected to
the public low-voltage power supply network that
supplies buildings used for domestic purposes.
Declaration: After the electromagnetic compatibility test, the essential performance
of SpO2 and PR meet the requirements as follow:
a) SpO2: Accuracy at 70%~100% is ±2%, at 0~69% is unspecified. Measuring
range is 0~100%.
b) PR: Accuracy is ±1% or ± 1 bpm, whichever is greater. Measuring range is
25bpm~250bpm.
Instructions on Environmental Aspects
Instructions for minimizing environmental impact during normal use.
1. Instructions on how to install the fingertip pulse oximeterin order to minimize the
ENVIRONMENTALIMPACT during its EXPECTED SERVICE LIFE;
Try to keep the integrity of the non-disposable packing material and put away the
packing materials for future use or put into the specified location where complying
with the rules and regulations of the local and the hospital. Avoid overusing the
cleaning reagents and other substances.
2. Instructions on how to use and maintain the fingertip pulse oximeter in order to
minimize the ENVIRONMENTAL IMPACT during its EXPECTED SERVICE LIFE;
Do not mix disinfecting solutions (such as bleach and ammonia) as this may
result in hazardous or poisonous gases or liquids. When there is a need to maintain,
please follow the instruction for use of follow the rules and regulations of the
hospital.
3. Consumption during NORMAL USE (e.g. energy, consumable materials/parts,
disposables, water, gasses, chemicals/reagents etc.);
During normal use of this device, it will consume electricity (battery). The
batteries shall be disposed following the rules. For cleaning or disinfection for the
machine, the water and ethanol will be used and the waste liquid shall be thrown
following the rules.
4. Emissions during NORMAL USE (e.g. WASTE water, WASTE consumable
materials, acoustic, energy, heat, gasses, vapours, particulates, HAZARDOUS
SUBSTANCES and other WASTE);
Consumption of the battery during use.
5. Information on the location within the device of HAZARDOUS SUBSTANCES,
radioactive sources and induced radioactive materials.
This product has no hazardous substances, such as radioactive sources or
induced radioactive materials.
Information for end of life management.
1. The location of components and parts within the device that contain stored
energy or pose other hazards that can result in an unacceptable risk to
disassembles or others and methods for controlling such risks.
The device uses an alkaline battery.May heat,explode or leak if
shotted,recharged,disposed of in fire or dissected.
2. The identity and location of hazardous substances requiring special handling and
treatments.
The battery is installed in the battery case.
3. Disassembly instructions sufficient for the safe removal of these hazardous
substances including radioactive sources and induced radioactive materials within
the monitor.
For other hazards that may result in unacceptable risk, the main concern is the
handing with battery.Do not store the battery in a high temperature environment
and store the battery in a cool, ventilated environment.
As for disposing or recycling of the device and device components at end of life,
follow local ordinances and recycling instructions regarding.
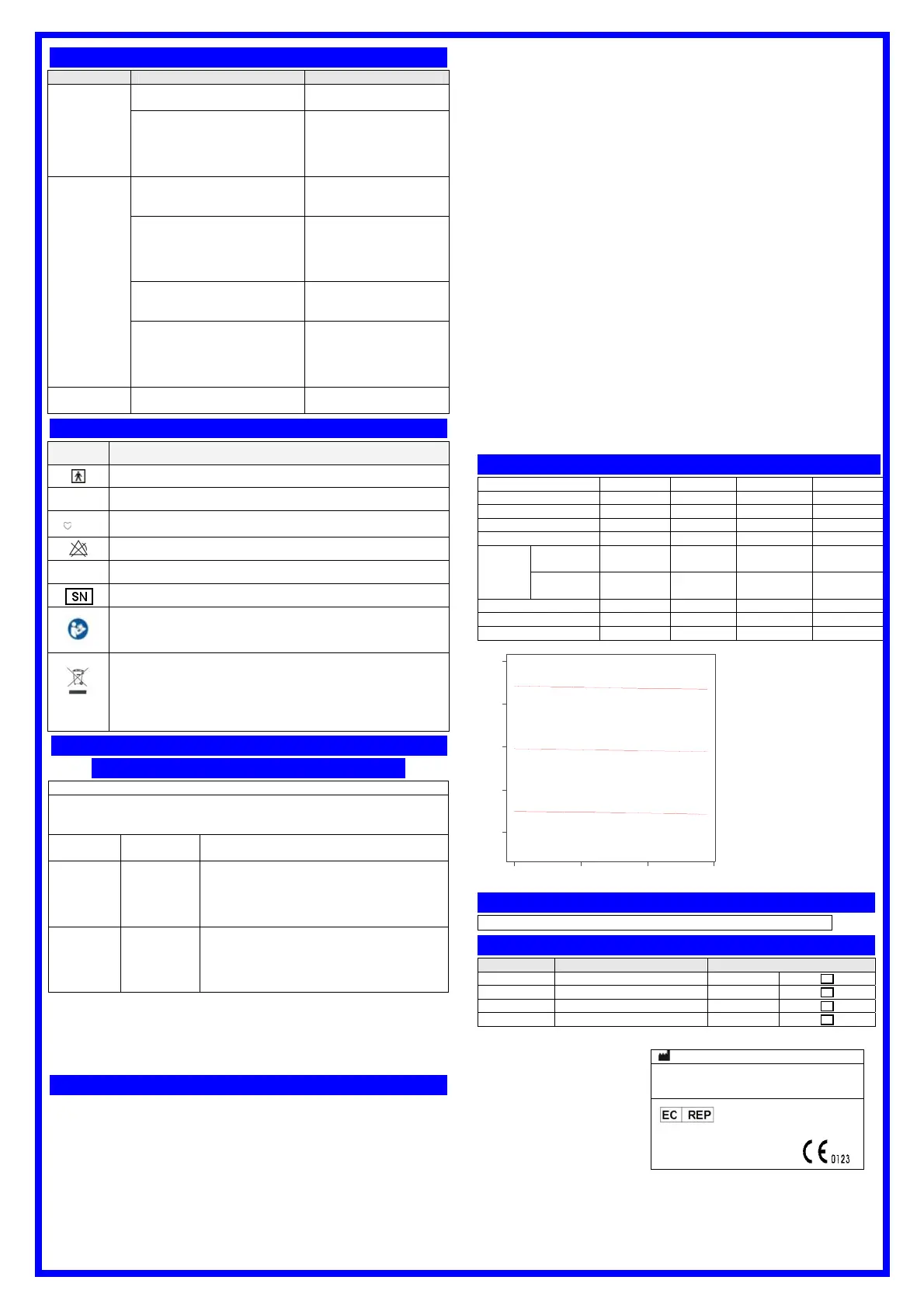
Clinical Trial Results
70-100 70-79 80-89 90-100
Count 325 83 87 155
Mean Bias -0.1692 -0.0843 -0.2874 -0.1484
Standard Deviation 1.4779 1.9140 1.6347 1.0678
Standard Error 0.0819 0.2100 0.1753 -0.086
95%CI
Lower
Bound
-0.3305 -0.5023 -0.6358 -0.3178
Upper
Bound
-0.0080 0.3336 0.0610 0.0210
Minimum 70.00 70 80 90
Maximum 99.00 79 89 99
Arms 1.4853 1.9043 1.6900 1.0746
70.00 80.00 90.00 100.00
SaO2
-4.00
-2.00
0.00
2.00
4.00
D
e
v
i
a
t
i
o
n
Applicable Models
M70, M70A, M70B
Packing List
NO. Item Quantity
1 Oximeter 1
2 AAA battery 2
3Cord1
4 User’s manual 1
GuangdongBiolightMeditech Co.,Ltd.
No.2 Innovation First Road, Technical
Innovation Coast, Hi-tech Zone Zhuhai
PEOPLE’S REPUBLIC OF CHINA
Shanghai International Holding Corp. GmbH
(Europe)
Eiffestrasse 80, 20537
Hamburg Germany
ALL RIGHTS RESERVED
PN: 22-025-0002

 Loading...
Loading...