Do you have a question about the BIOTRONIK Evia HF ProMRI and is the answer not in the manual?
Purpose of the Evia family pacemakers is to improve patient symptoms through bradycardia therapy.
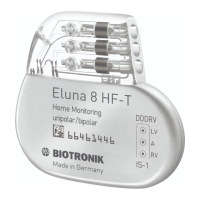
Introduces the Evia HF and HF-T triple-chamber devices and their availability.
Details available device variants and their corresponding NBG codes (DDDRV).
Highlights features for bradycardia diagnosis, therapy, and automatic functions like auto-initialization.
Discusses common complications like fluid accumulation, infections, and tissue reactions.
Lists potential technical malfunctions like lead dislocation, insulation defects, and battery depletion.
Details interference from external signals, device behavior during EMI, and static magnetic fields.
Covers contraindicated and risky procedures, and external defibrillation considerations.
Outlines recommended indications and known contraindications for pacemaker implantation.
Specifies permitted temperature ranges and storage location requirements for the device.
Describes the general implantation location on the body.
Lists the step-by-step procedure for implanting the device.
Defines the schedule for regular in-office follow-ups and Home Monitoring integration.
Explains how Home Monitoring supports follow-up and patient monitoring.
Provides a step-by-step procedure for conducting in-house follow-up using the programmer.
Details the available pacing modes for the Evia family, dependent on individual diagnosis.
Specifies parameters related to basic rate, night rate, AV delay, and VV delay.
Describes settings for AV hysteresis and repetitive AV hysteresis.
Explains the Vp suppression function to avoid unnecessary ventricular pacing.
Specifies acceptable variations for various device parameters.
Provides measurements for the device housing, including dimensions, volume, and mass.
Details electrical components, input values, pulse form, polarity, and housing shape.
Lists battery characteristics from manufacturers, including type, voltage, capacity, and usable capacity.
Explains the meaning of various symbols found on device labels, including manufacturing date, temperature limit, and sterile status.
Illustrates examples of uncoated/coated devices, NBG codes, screwdriver, header, and connector types.
| Telemetry | Wireless telemetry |
|---|---|
| Home Monitoring | Yes |
| Remote Monitoring | Yes |
| Therapy | Cardiac Resynchronization Therapy (CRT) |
| Capture Control | Automatic |
| Ventricular Sensing | Yes |
| MRI Compatibility | MR Conditional |











 Loading...
Loading...