Do you have a question about the BTL BTL-08 ABPM and is the answer not in the manual?
Welcomes users and explains the manual's purpose for understanding the BTL-08 ABPM system.
Lists clinical conditions for which ABPM is indicated, based on hypertension recommendations.
Lists patient groups or conditions where ABPM monitoring is not recommended or requires caution.
Details cases where the device should not be used and critical safety advice for operation.
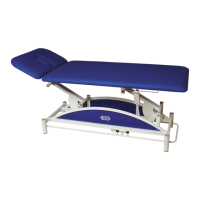
Describes the BTL-08 ABPM device, its components, memory capacity, and measurement method.
Explains how to use the START, MEDICATION, and DAY/NIGHT buttons for various functions like measurements, marking events, and shifts.
Details the meaning of various symbols and readings shown on the device's LCD screen for status and results.
Provides essential guidelines for patients and operators to ensure successful monitoring sessions and patient comfort.
Step-by-step instructions for establishing a wired connection between the ABPM recorder and a computer using an interface cable.
Outlines the sequential steps for conducting a typical 24-hour ABPM monitoring session, from preparation to data retrieval.
Describes the functions of the BTL-08 ABPM software for programming, data transfer, analysis, and reporting.
Details the different cuff sizes, their application, and important considerations for accurate measurements and patient comfort.
Lists the standard accessories included with the BTL-08 ABPM recorder package for complete functionality.
Specifies the minimum and recommended hardware and OS configurations for installing and running the BTL-08 ABPM software.
Provides detailed instructions for installing the BTL-08 ABPM SW Personal Edition on a computer.
Guides through the first launch of the software, including administrator login, settings, and user creation.
Detailed steps for physically connecting the ABPM recorder to the computer using the optoelectronic interface cable for data transfer.
Instructions for establishing a wireless connection between the ABPM recorder and the computer using Bluetooth technology.
Outlines the legal terms and conditions for using the BTL-08 ABPM software, including copyright and limitations.
Provides guidance on how to protect the device from damage, spills, and proper cleaning methods.
Details battery types, usage recommendations, charging, and the process for replacing batteries to ensure optimal performance.
Covers essential safety precautions, handling advice, warnings regarding electrical safety, biocompatibility, and hazardous materials.
Specifies the warranty periods and terms for the recorder unit, accessories, and software, along with common exclusions.
Lists the technical specifications of the BTL-08 ABPM, including power supply, display, data storage, measurement ranges, and accuracy.
Provides information on electromagnetic emission and immunity requirements, and recommended separation distances for RF equipment.
Contains the contact details, address, and website of the manufacturer, BTL Industries Ltd.
The BTL-08 ABPM is an ambulatory blood pressure recorder manufactured by BTL Industries Ltd., designed for 24-hour monitoring of blood pressure. It is a compact, lightweight device that operates with two AA size accumulators or batteries. The device's memory can store up to 600 measurements. It uses a stepwise pressure deflation method to ensure quality measurements, even in the presence of environmental factors. The BTL-08 ABPM SW (software) is developed by BTL Industries Ltd. and is protected by copyright laws and international treaty provisions.
The BTL-08 ABPM records blood pressure and pulse rate values, providing data to support diagnostic decisions by a qualified physician. It does not automatically provide a diagnosis. The device is intended for use in ambulatory blood pressure monitoring (ABPM) and is indicated for suspected white-coat hypertension, nocturnal hypertension, establishing dipper status, resistant hypertension, elderly patients, as a guide to antihypertensive drug treatment, Type 1 diabetes, hypertension of pregnancy, evaluation of hypotension, and autonomic failure.
The device features an LCD display, START, MEDICATION, and DAY/NIGHT buttons. The START button initiates a manual blood pressure measurement, displays battery voltage, and can be used to switch the device on or off. The MEDICATION button allows patients to mark events without starting a measurement, such as taking medicine. The DAY/NIGHT button, if enabled during programming, allows patients to mark times of going to bed and rising, or manually shift the measurement frequency period (day or night). Any button can be pressed to interrupt a blood pressure measurement, causing immediate cuff deflation.
The BTL-08 ABPM SW allows for programming the recorder, transferring collected data to a computer, and analyzing blood pressure profiles. It stores data in a database with user access control and offers graphical and tabular displays, editing features, statistical analysis, and a report generator.
| Brand | BTL |
|---|---|
| Model | BTL-08 ABPM |
| Category | Medical Equipment |
| Language | English |