7
CEpartner4U BV,Esdoornlaan 13
3951 DBMaarn, The Netherlands
Phone: +31.6.516.536.2
6
Codonics Inc.,17991 Englewood Dr.
Middleburg Heights OH, 44130 USA
This device complies with part 15 of the FCC Rules. Operation is subject to the
following two conditions: 1)This device may not cause harmful interference, and 2)
this device must accept any interference received, including interference that may
cause undesired operation.
This Class B digital apparatus complies with Canadian ICES-003. Cet appareil
numerique de la Classe B est conform a la norme NMB-003 du Canada.
This product is in conformity with the requirements of EC Council directive
93/42/EEC on the approximation of the laws of the Member States relating to
medical devices. This product satisfies the Class B limits of EN 60601-1 and
CISPR 11
.
Virtua
TM
Medical Disc Publisher
Made in the U.S.A.
Patents Pending
All Rights Reserve
d
MODEL / MODLE
VIRTU
A-2
ETL CLASSIFIED
CONFORMSTO
UL STD 60601-1
CERTIFIEDTO
CAN/CSA STD C22.2 NO.601.1
3125782
Windows® XP Embedded
XXXXX-XXX-XXX-XXX
XXX-XXXXX
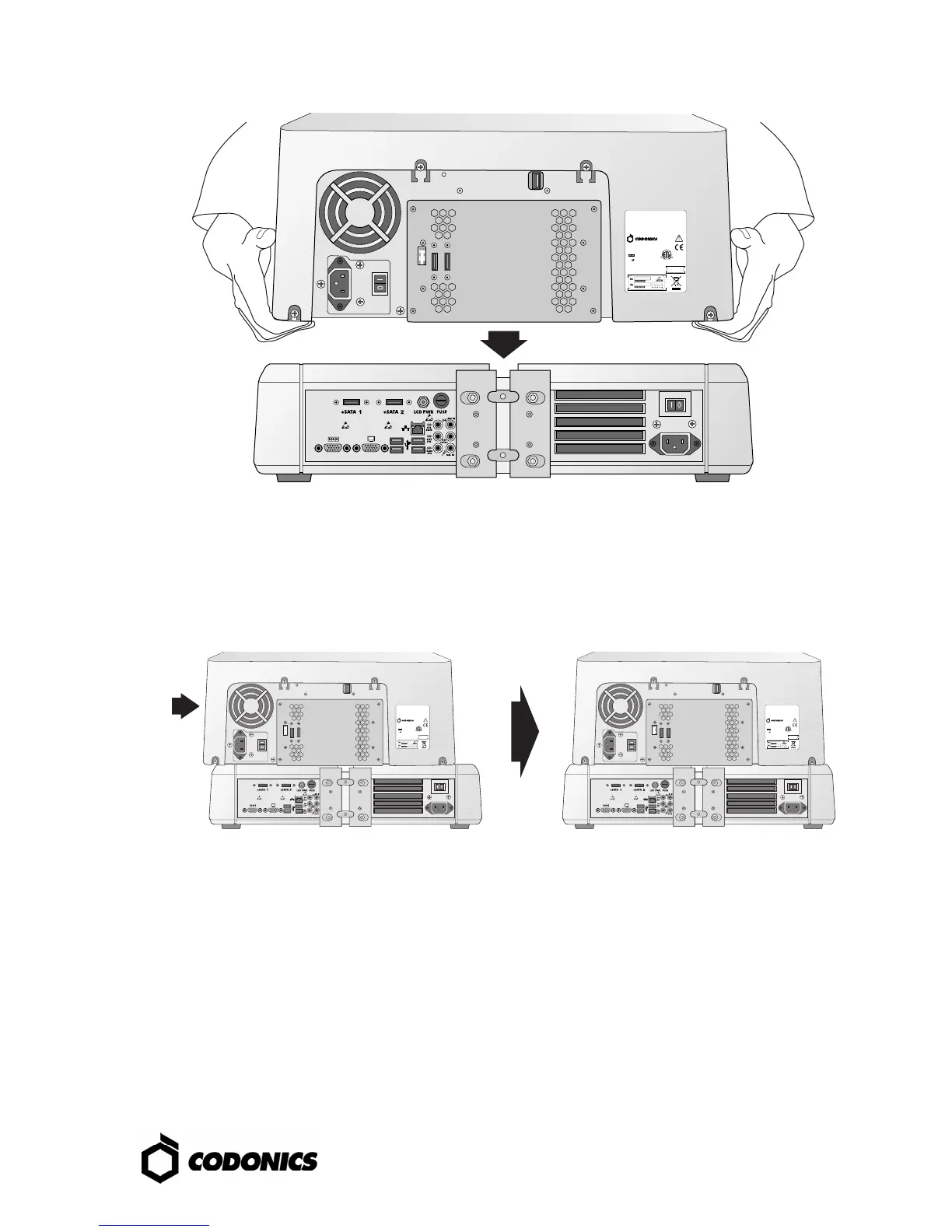
3. Place the Recorder on the Controller.
(Note: Offset the components to avoid pinching finger.)
CEpartner4UBV,Esdoornlaan 13
3951 DBMaarn,The Netherlands
Phone: +31.6.516.536.2
6
Codonics Inc.,17991 Englewood Dr.
Middleburg Heights OH, 44130 USA
This device complies with part 15 of the FCC Rules. Operation is subject to the
following two conditions: 1)This device may not cause harmful interference, and 2)
this device must accept any interference received, including interference that may
cause undesired operation.
This Class B digital apparatus complies with Canadian ICES-003. Cet appareil
numerique de la Classe B est conform a la norme NMB-003 du Canada.
This product is in conformity with the requirements of EC Council directive
93/42/EEC on the approximation of the laws of the Member States relating to
medical devices.This product satisfies the Class B limits of EN 60601-1 and
CISPR 11
.
Virtua
TM
Medical Disc Publisher
Made in the U.S.A.
Patents Pending
All Rights Reserve
d
MODEL / MODLE
VIRTU
A-2
ETL CLASSIFIED
CONFORMSTO
UL STD 60601-1
CERTIFIEDTO
CAN/CSA STD C22.2 NO.601.1
xxxxxxx
Windows® XP Embedded
XXXXX-XXX-XXX-XXX
XXX-XXXXX
CEpartner4UBV,Esdoornlaan 13
3951 DBMaarn,The Netherlands
Phone: +31.6.516.536.2
6
Codonics Inc.,17991 Englewood Dr.
Middleburg Heights OH, 44130 USA
This device complies with part 15 of the FCC Rules. Operation is subject to the
following two conditions: 1)This device may not cause harmful interference, and 2)
this device must accept any interference received, including interference that may
cause undesired operation.
This Class B digital apparatus complies with Canadian ICES-003. Cet appareil
numerique de la Classe B est conform a la norme NMB-003 du Canada.
This product is in conformity with the requirements of EC Council directive
93/42/EEC on the approximation of the laws of the Member States relating to
medical devices.This product satisfies the Class B limits of EN 60601-1 and
CISPR 11
.
Virtua
TM
Medical Disc Publisher
Made in the U.S.A.
Patents Pending
All Rights Reserve
d
MODEL / MODLE
VIRTU
A-2
ETL CLASSIFIED
CONFORMSTO
UL STD 60601-1
CERTIFIEDTO
CAN/CSA STD C22.2 NO.601.1
xxxxxxx
Windows® XP Embedded
XXXXX-XXX-XXX-XXX
XXX-XXXXX
4. Center the Recorder on the Controller.
 Loading...
Loading...