Do you have a question about the Enraf Nonius Sonopuls 490 and is the answer not in the manual?
Overview of the 4-series family, including Endomed 482, Sonopuls 490, and Sonopuls 492.
Explanation of various symbols used for warnings, safety, and device status indicators.
Crucial safety advice, warnings, and cautions for operating the device.
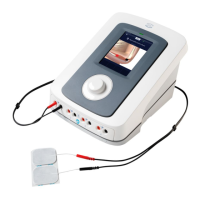
Identification and purpose of the main unit's physical components and connectors on page 7.
Lists of devices, treatment heads, and accessories included in the product package.
Steps for device inspection, connection, and initial setup.
Defines the target users and the intended medical applications of the device.
Lists medical conditions suitable for treatment and those where the device should not be used.
Guidance on using the touch screen interface, navigation bar, and controls.
Detailed description of electrotherapy current waveforms and ultrasound parameters.
Technical specifications for various electrotherapy currents, modulation, and surge programs.
Procedures for device cleaning, routine maintenance, and firmware updates.
Guidance on resolving common errors, alerts, and operational problems.
Detailed technical data, environmental conditions, and compliance with safety standards.
| Type | Ultrasound Therapy Device |
|---|---|
| Therapy Forms | Ultrasound |
| Treatment Time/Timer | 1 to 30 minutes |
| Number of Channels | 1 |
| Continuous and Pulsed Mode | Yes |
| Display | LCD |
| Programmable Positions | Yes |
| Multi-Frequency Treatment Head | Yes |
| Frequency | 1 MHz / 3 MHz |
| Ultrasound Head | 5 cm² |
| Duty Cycles | 20%, 50% |
| Pulse Ratio | 1:1 |
| Power Supply | 100-240 V |











 Loading...
Loading...