18
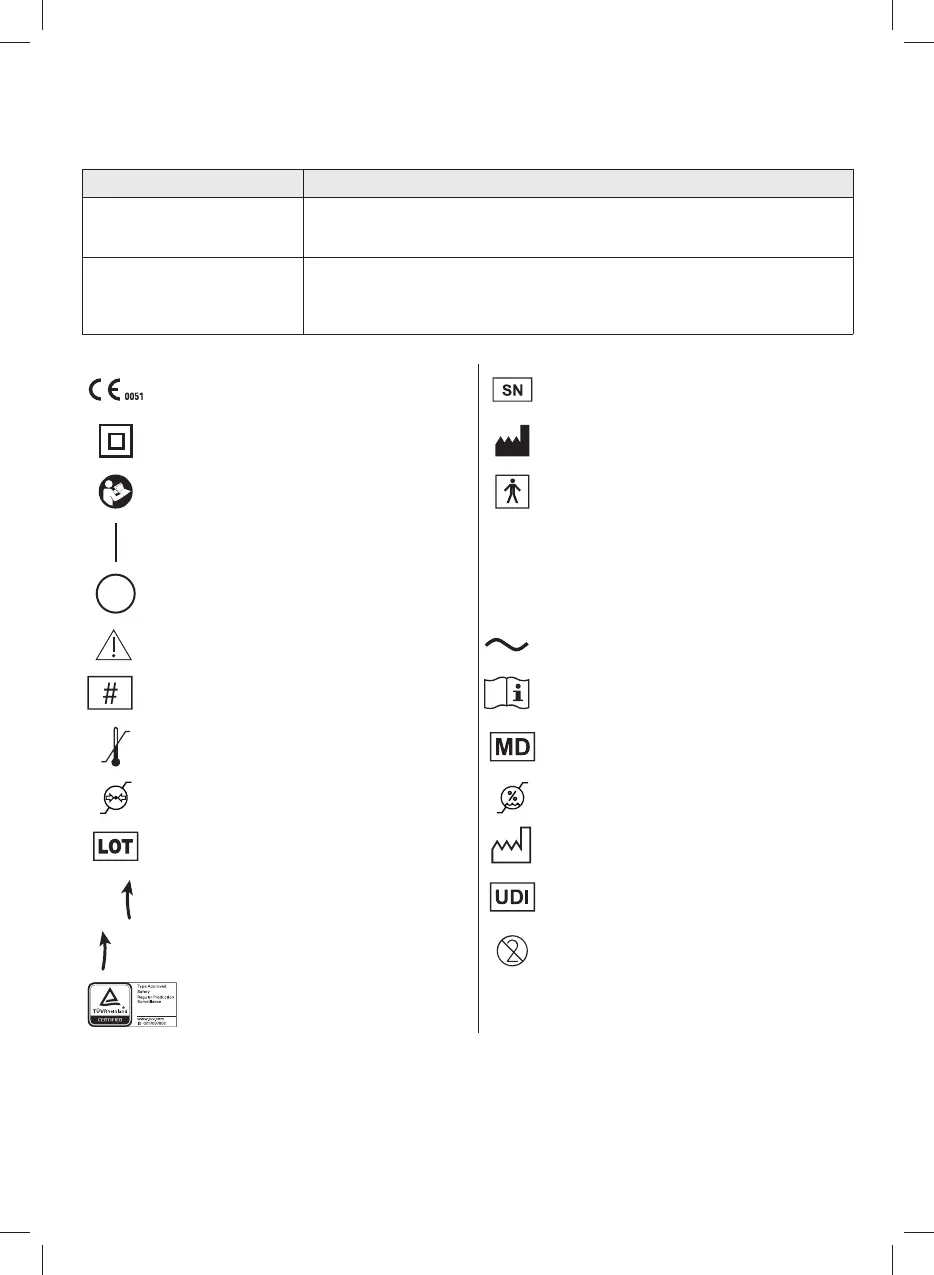
SYMBOLS ON DEVICE OR PACKAGING
Medical CE marking ref. regulation 2017/745 EU and
subsequent updates
Serial number of the device
Class II device Manufacturer
Before use: Caution check instructions for use Type BF applied part
Switched
'ON’
When switching o the unit, the switch stops
compressor operation on only one of the two
power phases.
IP21
Protection rating of the envelope: IP21.
(Protected against solid bodies larger than 12 mm.
Protected against access with a nger; Protected
against vertically falling drops of water).
Switched
'OFF’
Attention Alternating current
Model number See instructions for use
Temperature limits Medical device
Atmospheric pressure limits Moisture limits
Batch Code Production date
Greater vacuum Unique device identier
Less vacuum Single use
Quality mark
NOTIFICATION OF SERIOUS EVENTS
Serious events occurring in connection with this product must be reported immediately to the manufacturer or the competent
authority.
An event is considered serious if it causes or may cause, directly or indirectly, death or an unforeseen serious deterioration in a
person's state of health.
COUNTRY AUTHORITY
Ireland
Health Products Regulatory Authority
Kevin O’Malley House, Earlsfort Centre, Earlsfort Terrace, IE - Dublin 2
E-mail: devicesafety@hpra.ie
Malta
Medical Devices Unit Medicines Authority
Sir Temi Żammit Buildings, Malta Life Sciences Park
San Ġwann SĠN 3000
E-mail : devices.medicinesauthority@gov.mt
 Loading...
Loading...