20
APPLIANCE AND MATERIAL INFORMATION DEPENDING ON THE MODELS SUPPLIED
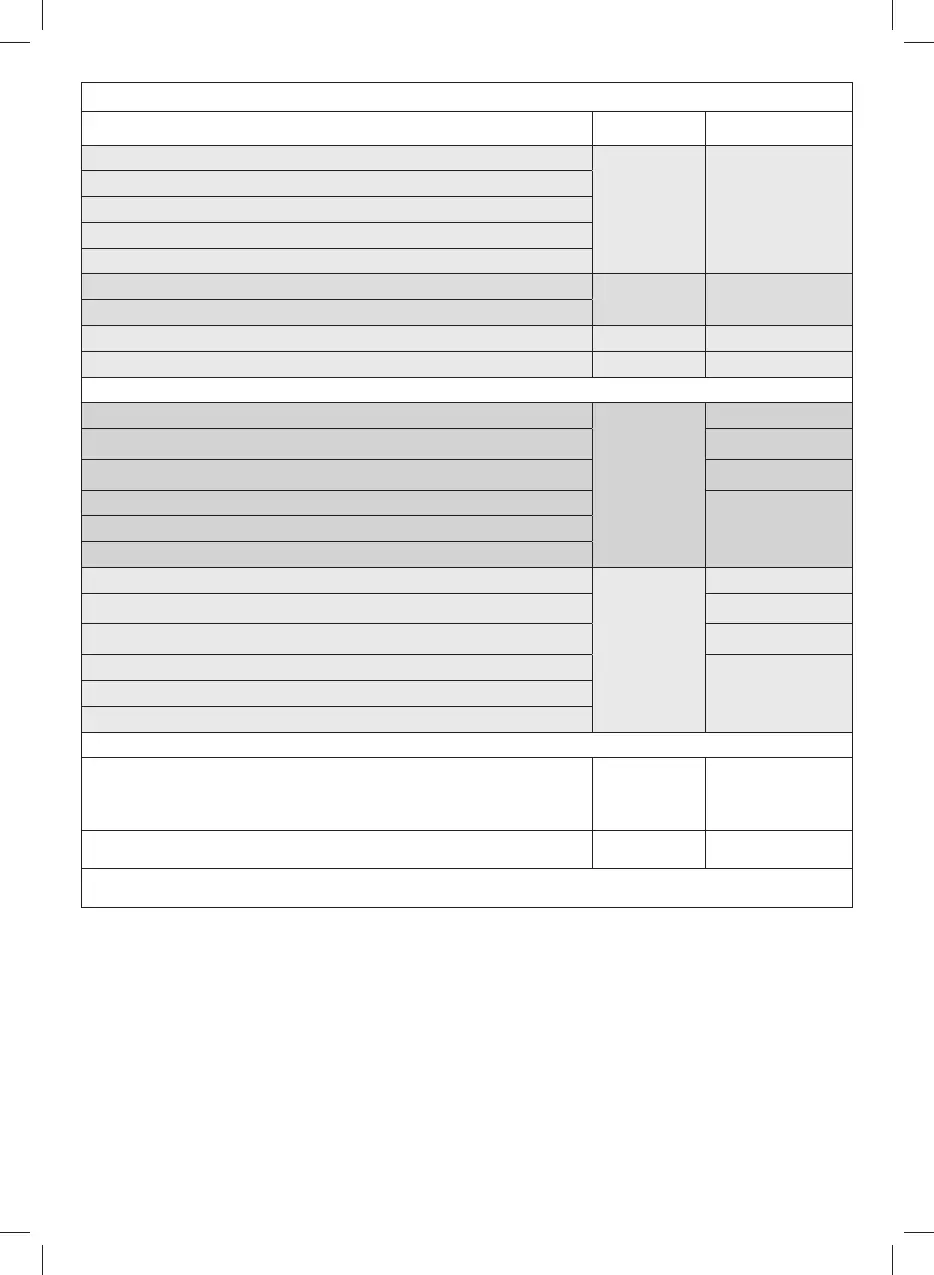
Nomenclature Model
Information on
materials
1)
ASPIR
ATOR
P1211EM/11l
P1211EM/13.5l
P1211EM/20l
2)
SWIT
CH
3)
V
ACUUM GAUGE
4)
V
ACUUM ADJUSTMENT KNOB
5)
AIR INLE
T
6a)
C
ONNECTION PIPE - Ø 13 x 7.5 mm L 1300 mm
A005 Silicone
6b)
C
ONNECTION PIPE - Ø 13 x 7.5 mm L 280 mm
6c)
C
ONNECTION PIPE - Ø 13 x 7.5 mm L 280 mm Single-patient multiple use A007 Silicone
6d)
C
ONNECTION HOSE - Ø 13 x 7.5 mm L 280 mm L 2000 mm (Single use) A009 PVC
7)
H
YDROPHOBIAL ANTIVIRAL/ ANTIBACTERIAL FILTER (SINGLE USE) (device not manufactured by FLAEM)
8) COLLECTION VESSEL
ASP-4
Polycarbonate
10) PROTECTIVE DEVICE
Polypropylene+
Silicone
11) VESSEL CLOSURE CAP
Polypropylene + ther-
moplastic elastomers
12) VESSEL “VACUUM” SOCKET
13) VESSEL “PATIENT” SOCKET
14) LID PROTECTION CAPS
9) COLLECTION VESSEL
ASP-2
Polycarbonate
10) PROTECTIVE DEVICE
Polypropylene+
Silicone
11) VESSEL CLOSURE CAP
Polypropylene + ther-
moplastic elastomers
12) VESSEL “VACUUM” SOCKET
13) VESSEL “PATIENT” SOCKET
14) LID PROTECTION CAPS
15) MANUAL ASPIRATED FLOW CONTROL - (disposable) (device not manufactured by FLAEM)
16)
ASPIRATOR CANNULA. (NOT SUPPLIED WITH THE DEVICE). The cannula is not supplied and is
not available as a spare part for the medical device. Any cannulae to be used with our aspirators
must be of a shape adaptable to the 'MANUAL ASPIRATED FLOW CONTROL' connection supplied
with the aspirator.
17) POWER SUPPLY CABLE
17a) PROTECTION SEAL
IMPORTANT NOTE: There is an identication label on the packaging, remove it and ax it in the spaces provided on page 2. When
replacing the Nebuliser and accessories, perform the same procedure.
 Loading...
Loading...