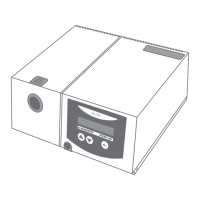
Intended Use
The TREND 300 is for treatment of nighttime sleep
apnea in patient weighing at least 30 kg.
This device is not intended for use with ventilator-
dependent patients.
The TREND 300 provides positive airway pressure in
two pressure levels.
This device is designed to be used with nasal breathing
masks equipped with an exhalation port to allow a con-
tinuous flow of air through the mask. Do not wear the
mask with the device turned off as rebreating of the air
occurs. Refer to the manufacturer´s instructions for the
mask and exhalation port.
Devices equipped with an AquaTREND accessory
humidifier provide humidification of the air delivered to
the patient.
Contraindications
The respiration therapy may be contraindicated for pati-
ents with the following pre-existing medical conditions:
■ Bullous lung diseases
■ Pneumothorax
■ Extremely low blood pressure
■ Pneumocephalus or pre-existing CSF leaks or head
trauma.
Acute sinus or middle ear infection may be an indication
to suspend the therapy. Contact your physician if you
have questions about this condition.
Devices equipped with an AquaTREND accessory
humidifier are not intended for use with medications,
aromatic oils or other substances added to the humidi-
fier water.
Not for use with patients whose supraglottic airways
have been bypassed.
8
Respiration Therapy Device TREND 300
Intended Use
 Loading...
Loading...