• DO NOT disassemble or attempt to repair this monitor or other
components. This may cause an inaccurate reading.
6.2 Storage
Keep your monitor in the storage case when not in use.
• Store your monitor in a clean, safe location.
Do not store your monitor:
• If your monitor is wet.
• In locations exposed to extreme temperatures, humidity, direct
sunlight, dust or corrosive vapors such as bleach.
• In locations exposed to vibrations or shocks.
6.3 Cleaning
• Do not use any abrasive or volatile cleaners.
• Use a soft dry cloth or a soft cloth moistened with neutral soap to
clean your monitor and wrist cuff, and then wipe them with a dry
cloth.
• Do not wash or immerse your monitor and wrist cuff in water.
• Do not use gasoline, thinners or similar solvents to clean your
monitor and wrist cuff.
6.4 Calibration and Service
• The accuracy of this blood pressure monitor has been carefully
tested and is designed for a long service life.
• It is generally recommended to have the unit inspected every two
years to ensure correct functioning and accuracy. Please consult
your authorised OMRON dealer or the OMRON Customer Service
at the address given on the packaging or attached literature.
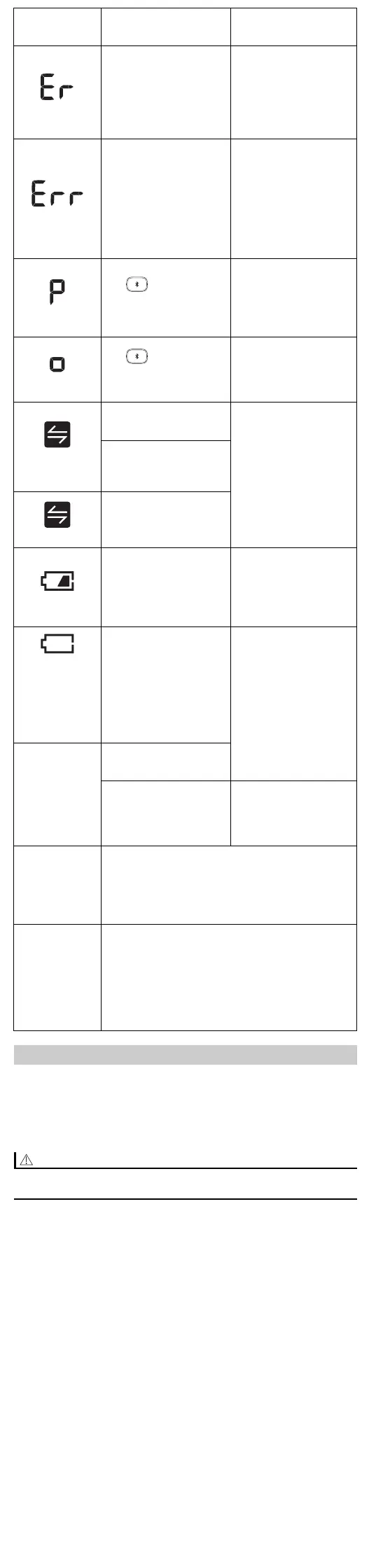
appears
The monitor is
malfunctioned.
Press the [START/
STOP] button again. If
“Er” still appears,
contact your OMRON
retail outlet or
distributor.
appears
The monitor cannot
connect to a smart
device or transmit data
correctly.
Follow the instructions
shown in the “OMRON
connect” app. If the
“Err” symbol still
appears after checking
the app, contact your
OMRON retail outlet or
distributor.
flashes
The button is
pressed and held to pair
with a smart device.
Flashes when pairing to
the smart device. Visit
the “Help” section in the
“OMRON connect” app
for pairing.
flashes
The button is
pressed to transfer your
readings.
Flashes when
transferring your
readings to the
“OMRON connect” app.
flashes
More than 24 readings
are not transferred.
Pair or transfer your
readings to the
“OMRON connect” app
so you can keep them
in memory in the app,
and this error symbol
disappears.
Your monitor is not
paired or not connected
with your smart device.
appears
30 readings are not
transferred.
flashes
Batteries are low.
Replacing all batteries
with 2 new alkaline
batteries is
recommended. Refer to
section 2.1.
appears or the
monitor is
turned off
unexpectedly
during a
measurement
Batteries are depleted.
Immediately replace all
batteries with 2 new
alkaline batteries. Refer
to section 2.1.
No power.
Nothing
appears on the
display of the
monitor.
Batteries are completely
depleted.
Battery polarities are not
properly aligned.
Check the battery
installation for proper
placement. Refer to
section 2.1.
Readings
appear too high
or too low.
Blood pressure varies constantly. Many factors
including stress, time of day, and/or how you
apply the wrist cuff, may affect your blood
pressure. Review sections 2.3 - 2.5 and chapter
3.
Any other
problems occur.
Press the [START/STOP] button to turn the
monitor off, then press it again to take a
measurement. If the problem continues, remove
all batteries and wait for 30 seconds. Then re-
install batteries.
If the problem still persists, contact your OMRON
retail outlet or distributor.
6. Maintenance
Display/
Problem
Possible Cause Solution
• These specifications are subject to change without notice.
• In the clinical validation study, K5 was used on 85 subjects for
determination of diastolic blood pressure.
• This monitor is clinically investigated according to the
requirements of EN ISO 81060-2:2014.
• IP classification is degrees of protection provided by enclosures in
accordance with IEC 60529. This monitor is protected against
solid foreign objects of 12.5 mm diameter and greater such as a
finger, and against oblique falling water drops which may cause
issues during a normal operation.
• This device has not been validated for use on pregnant patients.
Thank you for buying an OMRON product. This product is
constructed of high quality materials and great care has been taken
in its manufacturing. It is designed to give you every satisfaction,
provided that it is properly operated and maintained as described in
the instruction manual.
This product is warranted by OMRON for a period of 3 years after
the date of purchase. The proper construction, workmanship and
materials of this product is warranted by OMRON. During this period
of warranty OMRON will, without charge for labour or parts, repair or
replace the defect product or any defective parts.
The warranty does not cover any of the following:
A. Transport costs and risks of transport.
B. Costs for repairs and / or defects resulting from repairs done by
unauthorised persons.
C. Periodic check-ups and maintenance.
D. Failure or wear of optional parts or other attachments other than
the main device itself, unless explicitly warranted above.
E. Costs arising due to non-acceptance of a claim (those will be
charged for).
F. Damages of any kind including personal caused accidentally or
from misuse.
G. Calibration service is not included within the warranty.
Should warranty service be required please apply to the dealer
whom the product was purchased from or an authorised OMRON
distributor. For the address refer to the product packaging / literature
or to your specialised retailer. If you have difficulties in finding
OMRON customer services, contact us for information.
www.omron-healthcare.com
Repair or replacement under the warranty does not give rise to any
extension or renewal of the warranty period.
The warranty will be granted only if the complete product is returned
together with the original invoice / cash ticket issued to the
consumer by the retailer.
7. Specifications
Product category Electronic Sphygmomanometers
Product description Automatic Wrist Blood Pressure Monitor
Model (Code) RS3 Intelli IT (HEM-6161T-E)
Display LCD digital display
Cuff pressure range 0 to 299 mmHg
Blood pressure
measurement range
SYS: 60 to 260 mmHg
DIA: 40 to 215 mmHg
Pulse measurement
range
40 to 180 beats / min.
Accuracy
Pressure: ±3 mmHg
Pulse: ±5% of display reading
Inflation Automatic by electric pump
Deflation Automatic rapid deflation
Measurement method Oscillometric method
Transmission method
Bluetooth
®
low energy technology
Wireless communication
Frequency range: 2.4 GHz (2400 -
2483.5 MHz)
Modulation: GFSK
Effective radiated power: < 20 dBm
Operation mode Continuous operation
IP classification IP 22
Rating DC3 V 3.0 W
Power source 2 “AAA” alkaline batteries 1.5V
Battery life
Approximately 300 measurements (using
new alkaline batteries)
Durable period (Service
life)
5 years
Operating conditions
+10°C to +40°C / 15 to 90% RH (non-
condensing) / 800 to 1060 hPa
Storage / Transport
conditions
-20°C to +60°C / 10 to 90% RH (non-
condensing)
Weight
Approximately 85 g not including
batteries
Dimensions
Approximately 84 mm (w) × 62 mm (h) ×
21 mm (l)
(not including the wrist cuff)
Measurable wrist
circumference
13.5 to 21.5 cm
Memory Stores up to 30 readings
Contents
Monitor, 2 “AAA” alkaline batteries,
storage case, instruction manual, setup
instructions
Protection against
electric shock
Internally powered ME equipment
Applied part Type BF (wrist cuff)
Maximum temperature
of the applied part
Lower than +48°C
About a wireless communication interference
This product operates in an unlicensed ISM band at 2.4 GHz. In
the event this Product is used near other wireless devices such as
microwave and wireless LAN, which operate on the same
frequency band as this Product, there is a possibility that
interference may occur.
If interference occurs, stop the operation of the other devices or
relocate this Product away from other wireless devices before
attempting to use it.
8. Limited Warranty
• This device fulfils the provisions of EC directive 93/42/EEC
(Medical Device Directive).
• This blood pressure monitor is designed according to the
European Standard EN1060, Non-invasive sphygmomanometers
Part 1: General Requirements and Part 3: Supplementary
requirements for electromechanical blood pressure measuring
systems.
• Hereby, OMRON HEALTHCARE Co., Ltd., declares that the radio
equipment type HEM-6161T-E is in compliance with Directive
2014/53/EU.
• The full text of the EU declaration of conformity is available at the
following internet address: www.omron-healthcare.com
• This OMRON product is produced under the strict quality system
of OMRON HEALTHCARE Co., Ltd., Japan. The Core component
for OMRON blood pressure monitors, which is the Pressure
Sensor, is produced in Japan.
Important information regarding Electro Magnetic Compatibility
(EMC)
Correct Disposal of This Product (Waste Electrical & Electronic
Equipment)
9. Guidance and Manufacturer’s Declaration
Symbols description
Applied part - Type BF
Degree of protection against electric
shock (leakage current)
Ingress protection degree provided by
IEC 60529
CE Marking
Serial number
Temperature limitation
Humidity limitation
Atmospheric pressure limitation
To indicate generally elevated, potentially
hazardous, levels of non-ionizing
radiation, or to indicate equipment or
systems e.g. in the medical electrical
area that include RF transmitters or that
intentionally apply RF electromagnetic
energy for diagnosis or treatment.
Need for the user to consult this
instruction manual.
Indicates the correct positioning for the
monitor on the wrist
Measurable wrist circumference
Battery
Direct current
HEM-6161T-E manufactured by OMRON HEALTHCARE Co., Ltd. conforms to
EN60601-1-2:2015 Electro Magnetic Compatibility (EMC) standard.
Further documentation in accordance with this EMC standard is available at
www.omron-healthcare.com. Refer to the EMC information for HEM-6161T-E on
the website.
This marking shown on the product or its literature, indicates that it
should not be disposed of, with other household wastes at the end
of its working life.
To prevent possible harm to the environment or human health from
uncontrolled waste disposal, please separate this product from
other types of wastes and recycle it responsibly to promote the
sustainable reuse of material resources.
Household users should contact either the retailer where they purchased this
product, or their local government office, for details of where and how they can
return this item for environmentally safe recycling.
Business users should contact their supplier and check the terms and conditions of
the purchase contract. This product should not be mixed with other commercial
waste for disposal.
IP XX
The Bluetooth
®
word mark and logos are
registered trademarks owned by the Bluetooth
SIG, Inc. and any use of such marks by
OMRON HEALTHCARE Co., Ltd. is under
license. Other trademarks and trade names are
those of their respective owners.

 Loading...
Loading...