© 2018-2021 LifeScan
IP Holdings, LLC
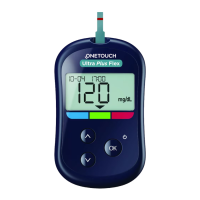
LifeScan self-test blood glucose
monitoring devices conform to
the following EU Directives:
IVDD (98/79/EC):
Blood Glucose Meter,
Test Strips, and
Control Solution
MDD (93/42/EEC):
Lancets
Lancing Device
Contact OneTouch
®
Customer Service at Tel:
HK: (852) 2735 8262, Mon - Fri
9am 12.30pm & 2.00 - 5.30pm.
MY: 1800 88 3338. Available
Monday to Friday, 9.00 a.m.
to 5.00 p.m. (except Public
Holidays). SG: +65 64157282
(Mon-Fri, 9AM – 5PM except
Public Holidays).
contact.sg@onetouch.com
Rev. Date: 06/2021
AW 07135706A
IMPORTED BY/DISTRIBUTED BY
DKSH Singapore Pte Ltd: 47
Jalan Buroh #09-1, Singapore
619491
Patent https://www.onetouch.com/patents
Legal Manufacturer:
LifeScan Europe GmbH
Gubelstrasse 34
6300 Zug
Switzerland
Imported by and Authorised
Representative:
DKSH Malaysia Sdn Bhd
B-11-01, The Ascent, Paradigm
N0. 1 Jalan SS 7/26A, Kelana Jaya
47301 Petaling Jaya
Selangor Darul Ehsan, Malaysia
HK0021900071
Complies with
IMDA Standards
N1046-21
 Loading...
Loading...