38
Specifications
Product Description
Model
Display
Measurement Method
Oscillometric Method
Wrist-type Fully Automatic
Blood Pressure Monitor
BSP-21
LCD Digital Display
Size:45.0mm×30.0mm
Pressurization
Memory
120 Memories
Automatic Pressurization
Function
Irregular Heartbeat Detection
WHO Classification Indicator
Last 3 Results Average
Low Battery Detection
Automatic Power-Off
Measurement Range
Pulse
Measurement Accuracy
Pressure
Pressure
Pulse
30 to 180 Beats/Minute
0mmHg ~ 300mmHg
±3mmHg or ±2% of the reading above
200 mmHg
±5%
39
Specifications
Humidity
Pressure
Power Source
Battery Life
Unit Weight
Unit Dimensions
Cuff Circumference
Operating Environment
Temperature
2 Alkaline Batteries Size AAA
Approximately 2 m at 3 tests per dayonths
Approx. 114g (4.02 oz.)( Excluding Battery)
Approx.75 (W)×300(L) mm
Fits wrist circumference 13.5-21.5 cm(5.3 -8.5 )" "
Storage Environment
Humidity
Classification
Temperature
Approx. 84 x 64 x 29mm
(3.31" x 2.52" x 1.14" )(L x W x H)
Specifications are subject to change without notice.
10 ~ 40 (50 ~104 )℃ ℃ ℉ ℉
15% ~ 90% RH
Atmospheric Pressure
15% ~ 90% RH
-20 ~55 (-4 ~131 )℃ ℃ ℉ ℉
Internal Powered Equipment,Type BF .
Ingress Protection
Rating
IP22
35
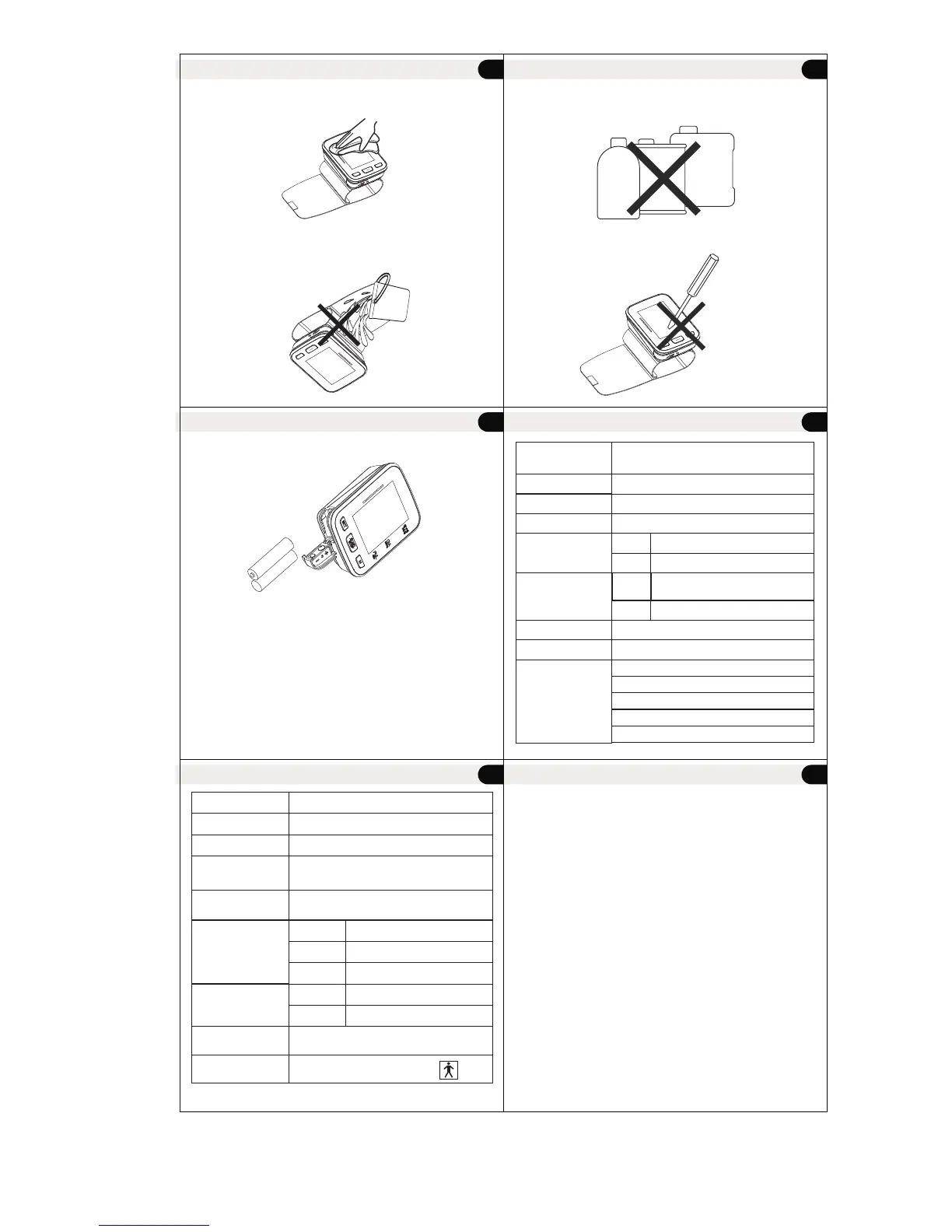
Maintenance
3. When cleaning the unit, use a soft fabric and lightly wipe with
mild detergent. Use a damp cloth to remove dirt and excess
detergent.
4. Do not soak cuff in water! Cuff Cleaning: Apply a small
amount of rubbing alcohol to a soft cloth to clean cuff's
surface. Use a damp cloth (water-based) to wipe clean.
Allow cuff to dry naturally at room temperature.
S
YS
D
IA
PL
U
M
S
ET
mm
Hg
m
m
H
g
/mi
n
ST
AR
T
ST
OP
SY
S
D
IA
P
L
U
M
SE
T
m
mH
g
mm
Hg
/
min
START
S
TO
P
36
Maintenance
5. Do not use petrol, thinners or similar solvents.
6. Remove batteries when not in operation for an extended
period of time.
S
Y
S
DIA
P
L
U
M
SET
m
m
H
g
mm
H
g
/m
i
n
ST
AR
T
S
TO
P
37
Maintenance
7. Do not disassemble product.
8. It is recommended the performance should be checked every
2 years.
9. Expected service life: Approximately three years at 10 tests
per day.
40
S
pe
c
i
f
ica
t
i
on
s
This Blood Pressure Monitor complies with the European
regulations.This blood pressure monitor also complies with
mainly following standards (included but not limited):
Safety standard:
EN 60601-1 Medical electrical equipment part 1:
General requirements for safety
EMC standard:
EN 60601-1-2 Medical electrical equipment part 1-2:
General requirements for safety- Collateral standard:
Electromagnetic compatibility- Requirements and tests
Performance standards:
EN 1060-1 Non-invasive sphygmomanometers -
General requirements
EN 1060-3 Non-invasive sphygmomanometers -
Supplementary requirements for
electromechanical blood pressure measuring systems.
EN 1060-4 Non-invasive sphygmomanometers -
Test procedures to determine the overall system accuracy
of automated non-invasive sphygmomanometers.

 Loading...
Loading...