55
<Example 2>
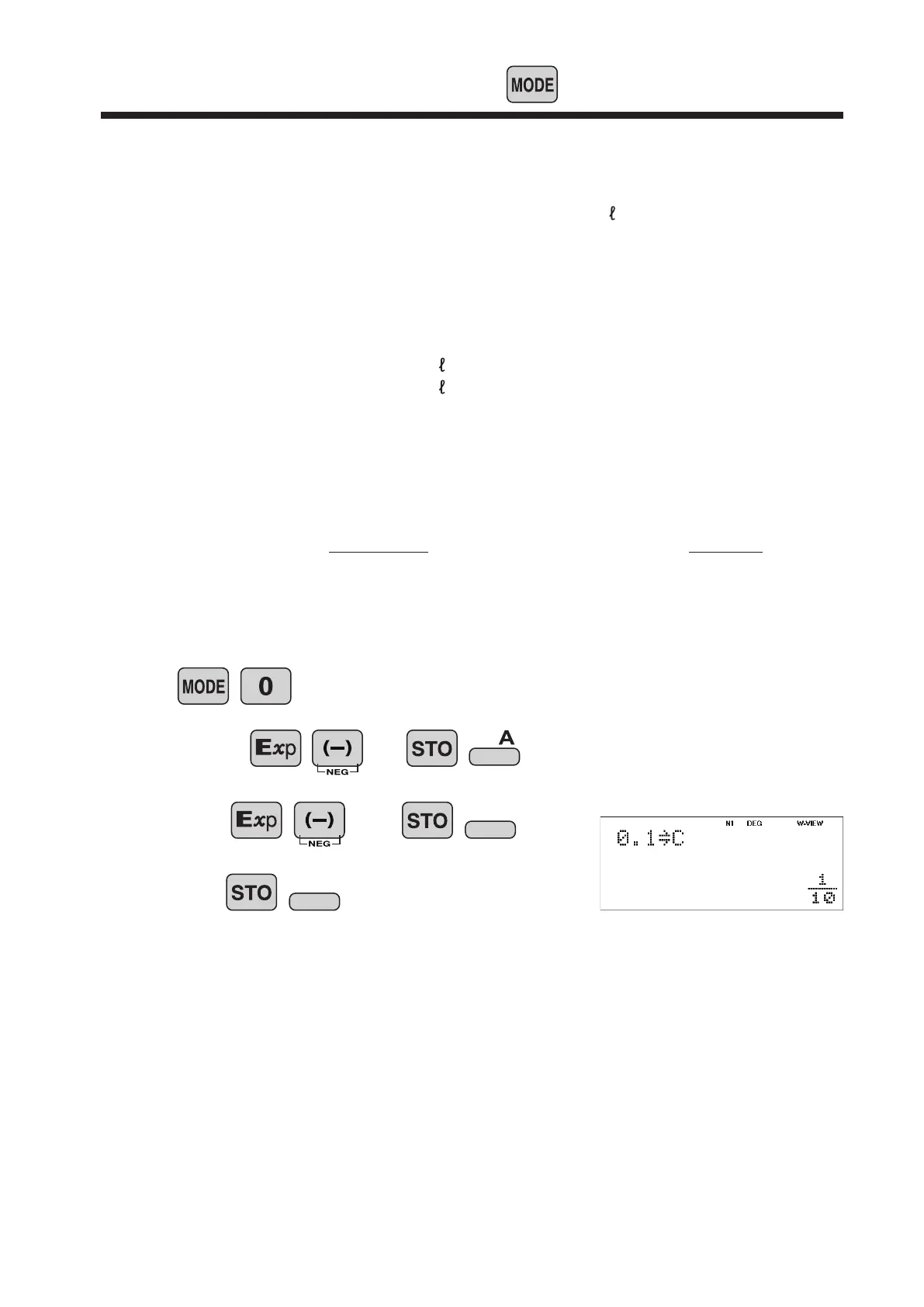
Let the acetic acid concentration be c (= 0.1 mol / ), and the hydrogen ion
concentration be
x
.
(1)
Solve the following quadratic equation to find the hydrogen ion concentration x:
x
3
+ K
a
x
2
- (cK
a
+ K
w
)x - K
a
K
w
= 0
where
K
a
= 2.75 x 10
-5
[mol / ] (
ionization equilibrium constant of acetic acid
)
K
w
= 1.0 x 10
-14
[mol / ] (ionic product of water)
(2) Use the result of (1) to find the pH (= - log x) of acetic acid.
Save constants
(1)
pH = - log x (x>0)
2.75 5
Operation
1.0
0.1
14
B
C
Display
Polynomial equation
(This example is for EL-W516T only.)
(NORMAL)
 Loading...
Loading...











