Do you have a question about the Storz DUOLITH SD1 R-SW and is the answer not in the manual?
Explains DANGER, WARNING, CAUTION, ATTENTION, and NOTE symbols used in the manual.
Outlines requirements for proper use, user qualifications, and applications.
Advises on connecting potential equalisation and handling electrical connectors safely.
Covers safe practices during patient treatment, observation, and approved therapies.
Recommends ear protection to minimise exposure to noise.
Discusses EMC compliance, potential interference, and installation guidelines.
Covers ventilation, storage, transport safety, and disposal considerations.
Explains the device mechanism and lists its intended uses.
Lists conditions where device use is prohibited and provides critical safety warnings.
Specifies user qualifications, abilities, and essential training for operating the device.
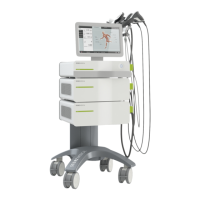
Provides a visual overview of the DUOLITH® SD1 R-SW system.
Identifies various device components from the back view.
Lists included accessories and guides on checking the device upon arrival.
Covers professional installation and correct placement of the device.
Details attaching and adjusting the handpiece holder for convenient storage.
Covers connecting to mains power, potential equalization, and voltage checks.
Guides on changing voltage settings and connecting the power cable.
Outlines steps before transport, including removing accessories and securing parts.
Identifies the standby switch and its role in controlling connected elements.
Details the rear connectors (HDMI, USB, Audio, LAN) on the control module.
Describes the USB connector and foot switch connector on the rear of the R-SW module.
Identifies module controls and lists handpieces compatible with the R-SW module.
Explains the handpiece's control and display elements for standalone operation.
Describes the V-ACTOR handpiece for vibration therapy and its shock transmitter heads.
Details the four connectors on the R-SW module for attaching handpieces.
Guides on connecting the air hose, suction cup, and filter for VACU-ACTOR.
Explains how to connect the optional foot switch to the R-SW module for US image control.
Provides steps to turn on the DUOLITH® SD1 R-SW using the main and standby switches.
Explains operation via touch screen and direct control using the handpiece.
Details the division of the user interface into different areas for displaying information.
Explains selecting operating modes and setting treatment parameters like energy and pulse count.
Details setting parameters and viewing pulse count and energy output.
Covers setting intensity, time limit, frequency, and mode for VACU-ACTOR.
Shows how to track contact intensity and indicates Skin Touch function status.
Provides crucial warnings about accidental shock triggering and excessive pressure.
Explains how to access indications via the menu bar (Orthopaedics, Patients, VAS).
Describes the bottom navigation bar for accessing software updates, options, and service.
Introduces touch screen interaction and password protection.
Details the steps for setting up and removing a password for the display.
Explains how to access and adjust device settings, screen brightness, and volume.
Guides on switching modes and setting treatment parameters manually or via indications.
Explains how to access and load indications, either all or specific to a zone.
Details list navigation and viewing magnified treatment photos.
Guides on loading treatment steps and associated patient records.
Details how to set parameters, name, and save custom indications.
Covers copying, deleting, and editing user-created indications.
Explains adding notes and loading images/videos to indications.
Covers creating, deleting, or editing individual treatment steps within an indication.
Guides on accessing and selecting stored patient records from a list.
Explains how to view detailed treatment parameters used for a specific patient.
Guides on editing patient data and assigning treatment parameters.
Details the process of creating a new patient record with name, date of birth, and ID.
Covers exporting data, deleting records, and resetting the shock counter.
Guides on updating software and connecting an external display.
Explains how to configure DICOM settings, requiring an upgrade kit.
Covers accessing the DICOM worklist and saving/printing DICOM images.
Explains how to configure the GDT interface for data exchange with surgery EDP systems.
Guides on initiating GDT requests and sending data back to the surgery system.
Illustrates the GDT transfer process, sending supplemented data back to the surgery system.
Introduces Visible Body as an interactive 3D atlas for representing body systems and regions.
Details how to launch the Anatomy Atlas application.
Explains how to select and deselect body systems within the submenu.
Describes the functions of toolbar symbols for navigation and interaction.
Guides on marking regions and accessing application settings.
Provides instructions on how to close the Visible Body application.
Covers activating Skin Touch and setting R-SW/V-ACTOR treatment parameters.
Details startup procedures and essential functional checks for the R-SW module.
Specifies default settings and critical safety information for treatment.
Offers safety cautions and guides on applying coupling agents for handpieces.
Refers to handpiece manual and lists VACU-ACTOR safety cautions.
Lists important safety warnings for using the VACU-ACTOR, including application areas and duration.
Guides on operating VACU-ACTOR using the foot switch or touch screen.
Lists common status messages and their corrective actions.
Provides a table of fault descriptions, causes, and corrective actions for system issues.
Covers general hygiene, electrical hazards, and cleaning procedures for device parts.
Details thorough cleaning and disinfection procedures for R-SW and V-ACTOR handpieces.
Refers to transmitter cleaning manual and outlines VACU-ACTOR part preparation.
Provides instructions for cleaning and disinfecting the polycarbonate suction cup.
Guides on overhauling the handpiece and replacing device fuses.
Covers regular checks, disposal, and authorized repair procedures.
Lists the average expected service life for the device, handpieces, and suction cup.
Provides a comprehensive list of available accessories and their part numbers.
Details operating modes, pressure, voltage, frequency, power, and environmental requirements.
Lists handpiece specs and explains device type plate details.
Lists applicable standards (EN 60601) and device compliance for safety and performance.
Details electromagnetic compatibility guidelines and emission characteristics.
Covers immunity tests (ESD, transients, surges) and recommended electromagnetic environments.
Specifies immunity levels for RF interference and recommended safety distances for portable equipment.
Provides a table to calculate recommended safety distances based on transmitter power.
Identifies labels on the trolley column, including potential equalization and type plate.
Details the labels and connectors on the right and rear sides of the R-SW module.
Identifies the manual compliance label on the handpiece and packaging transport conditions.
Outlines warranty terms for the device and accessories, and service contact information.
Guides on connecting the ultrasound probe to the DUOLITH touch screen.
Details how to disconnect both Telemed ultrasound and Smart US probes.
Explains switching on the module and references the separate ultrasound manual.
| Technology | Radial Shock Wave |
|---|---|
| Manufacturer | Storz Medical |
| Frequency | 1 - 21 Hz |
| Device Type | Extracorporeal Shock Wave Therapy (ESWT) Device |
| Application | Orthopedics, Sports Medicine, Rehabilitation |
| Display | Integrated touchscreen |
| User Interface | Touchscreen interface |
| Power Supply | 100 – 240 V / 50/60 Hz |
| Treatment Heads | Interchangeable Focused and Radial Applicators |
| Portability | Mobile unit with integrated compressor |