700000890732 Rev-AE EN
www.stryker.com 3
SINUS HEAD AND NECK
• Ethmoidectomy/
sphenoethmoidectomy
• Soft tissue shaving
• Septoplasty • Rhinoplasty
• Endoscopic
dacryocystorhinostomy
(DCR)
• Removal of fatty tissue
in the maxillary and
mandibular regions of the
face
• Frontal sinus trephination
• Transsphenoidal
procedures
• Polypectomy
• Antrostomy
• Frontal sinus drill-out
• Septal spurs removal
Contraindications
None known.
Description
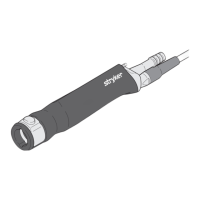
When used with a variety of accessories, the Stryker Pello
Microdebrider handpiece is used to cut and remove soft and
osseous tissue/bone. The handpiece features a quick locking
mechanism for accessory insertion. The handpiece is designed to
provide irrigation and suction to the accessory tip.
The CORE 2 console powers the handpiece and provides irrigation
to the accessories.
The footswitch provides control of the handpiece and irrigation
pump.
Safety Directives
WARNINGS:
• Only healthcare professionals trained and experienced in the
use of this device should use this equipment.
• Before using this equipment, or any component compatible with
this equipment, read and understand the instructions for use.
Pay particular attention to safety information. Become familiar
with equipment components prior to use.
• The healthcare professional performing any procedure is
responsible for determining the appropriateness of this
equipment and the specific technique used for each patient.
Stryker, as a manufacturer, does not recommend surgical
technique.
 Loading...
Loading...