Do you have a question about the Therabody TheraFace PRO and is the answer not in the manual?
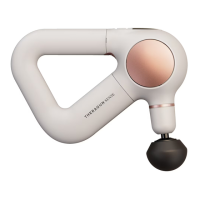
A 4-in-1 portable device offering microcurrent, LED, and cleansing treatments for well-being.
Designed to reduce tension, relax facial muscles, and improve skin appearance through gentle stimulation.
Percussive therapy tailored for facial needs, reducing tension and relaxing facial muscles.
Base gel for TheraFace PRO microcurrent treatments, ensuring effective and safe therapy.
Firms and tightens skin with microcurrents, improving muscle tone and facial contour.
Removes accumulated cosmetics, sebum, and impurities for deep facial cleansing.
Red light therapy reduces wrinkles around the eyes.
Blue light helps reduce mild to moderate acne.
Reduces wrinkles, provides therapeutic warming, and relieves tension.
Functions of the Power, Percussive Therapy, and Attachment Ring buttons.
Magnetic connection for easy attachment and removal of rings.
Attachments for percussive therapy: Flat, Cone, and Micro-point.
Optional rings for hot and cold therapy with multiple settings.
Press and hold the ON/OFF button to power on the device and activate the OLED screen.
Press and hold the ON/OFF button again to switch the device off.
The device automatically shuts off after 10 minutes of inactivity.
Steps include cleaning face, attaching ring, turning on, choosing settings, and starting.
Instructions on selecting, attaching, and operating the percussive attachments.
Guide to attaching and using the LED rings with different light options.
Steps for using the microcurrent ring, including gel application and intensity adjustment.
Information on the TheraOne Conductive Gel as a primer for microcurrent treatments.
Use as directed, avoid eye contact, test gel, reapply gel for glide.
Recommended order for applying toner, serum, moisturizer, and SPF after treatment.
Clean your face with a warm, damp washcloth after treatment.
Follow maintenance instructions to ensure continued proper device function.
Wipe device and attachments with a damp cloth or alcohol-free wipe.
Device intended for face, neck, upper chest. Consult physician for medical conditions.
Contraindications for use including epilepsy, cardiac issues, and electronic implants.
Warnings for minors, medical concerns, medications, and post-facial surgery.
Avoid recent surgery, facial treatments, breakouts, facial hair, and specific body areas.
Do not use with skin rash, open wounds, pacemakers, epilepsy, pregnancy, or tumors.
Avoid recent treatments, breakouts, facial hair, eyeballs, and broken skin; do not use retinol.
Avoid use with skin rash, wounds, pregnancy, cancer, epilepsy, or light-sensitive medications.
Use due care with recent treatments, breakouts, hypertension, and avoid specific body areas.
Do not use with skin rash, wounds, acne, bone fracture, heart/liver/kidney disease, or implants.
Avoid recent treatments, breakouts, facial hair, eyeballs, and broken skin.
Do not use with skin rash, wounds, blisters, or tumors.
Avoid recent treatments, breakouts, and facial hair.
Avoid recent treatments, breakouts, facial hair, eyeballs, and broken skin.
Do not use with skin rash, wounds, blisters, or tumors.
Use only instructed, recommended attachments, accessories, and parts. Do not self-maintain.
Device is not for children. Supervise children if they use the device.
Covers charging, overcharging, battery handling, damage, and disposal warnings.
Covers short circuits, storage, disposal, disassembly, and leakage precautions.
Covers overheating, service, water exposure, thermal limiters, plugs, and environment.
Covers specific risks associated with device usage.
Labels indicating compliance (CE, RoHS, WEEE, FCC) and handling (Read Manual).
Labels for 'Keep Dry' and 'Type BF Applied Part'.
Details on power source, indicator light, and housing materials.
Specifications for operating and storage environments, and atmospheric pressure.
Details on site, modes, intensity, timer, indicator, and housing materials.
Covers current, pulse width, charge, power density, and light power.
Frequency settings for percussive therapy: 1750, 2100, 2400 rpm.
Device is Class II with Type BF applied part, complying with IEC60601-1.
Device is compliant with Medical EMC Standard (IEC60601-1-2).
Device materials are biocompatible for intended use, complying with ISO standards.
Avoid using the device near devices with Electromagnetic Interference (EMI).
Device complies with FCC Part 15, subject to conditions to avoid harmful interference.
Equipment tested and compliant with Class B digital device limits for residential installations.
Measures to resolve radio or television reception interference.
Device uses RF energy internally; emissions are low and unlikely to cause interference.
Complies with harmonic emissions standards for domestic establishments.
Complies with voltage function and flicker emissions standards.
Immunity levels for electrostatic discharge and transient/bursts.
Immunity to surges and voltage variations on power supply lines.
Immunity to power frequency magnetic fields, with guidance on commercial/hospital environments.
Immunity to conducted RF energy, with recommended separation distances.
Immunity to radiated RF energy, with guidance on frequency ranges and interference.
Recommended separation distances for RF equipment to maintain safe operation.
Information on how to obtain full warranty details and request a copy.
Applicant certifies product conformity with harmonized standards and Council Directives.
Lists specific harmonized standards the product conforms to.
Lists relevant EU Council Directives the product complies with.
| Display | LED |
|---|---|
| Proper use | Face |
| Easy to use | Yes |
| Massage types | Combo massage, Percussive massage |
| Product color | Black |
| Number of speeds | 3 |
| Battery life | 120 min |
| Battery type | Built-in |
| Power source type | Battery |
| Quantity per pack | 1 pc(s) |
| Country of origin | China |
| Depth | 206 mm |
|---|---|
| Width | 183 mm |
| Height | 75 mm |
| Weight | 475 g |
| Package type | Box |
| Package depth | 325 mm |
| Package width | 198 mm |
| Package height | 235 mm |
| Package weight | 809 g |