Heat
11
1.13 Experiment 13
How water particles unite
Fill the atomizer with water. Spray the water onto a window or blackboard
from a distance of approximately 30 cm. The tiny particles from the atom-
izer form a barely visible water cloud, but on the sprayed surface they unite
after a while and form drops of increasing size.
Materials: 1 atomizer (6)
Additionally: some water
2 Underlying principles
Heat and thermometer
When solid bodies, liquids or gases are heated their volume (the amount
of space that they occupy) generally increases. For example, when warmed
by 1 degree Celsius (= 1 Kelvin), 1 litre of water and 1 litre of mercury both
expand by 0,2 millilitre (= 0.2 cubic centimetre), alcohol by 1.1 millilitre
(= 1.1 cubic centimetre).
When cooled, the volume decreases again. However, if water (under nor-
mal pressure) is cooled, its volume only decreases until it reaches 4 °C (Cel-
sius). If the water is cooled further to 0 °C its volume increases again. When
water at 0 °C changes to ice at 0 °C there is a sudden increase in volume.
This they cool and solidify.
Temperatures are measured using thermometers. Liquid thermometers
consist of a liquid-filled container (the bulb) connected to a very narrow
tube. If the temperature rises, the liquid will expand. The level of the liquid
in the tube at any given time can be read from a scale. Thermometers are
usually filled with alcohol or mercury.
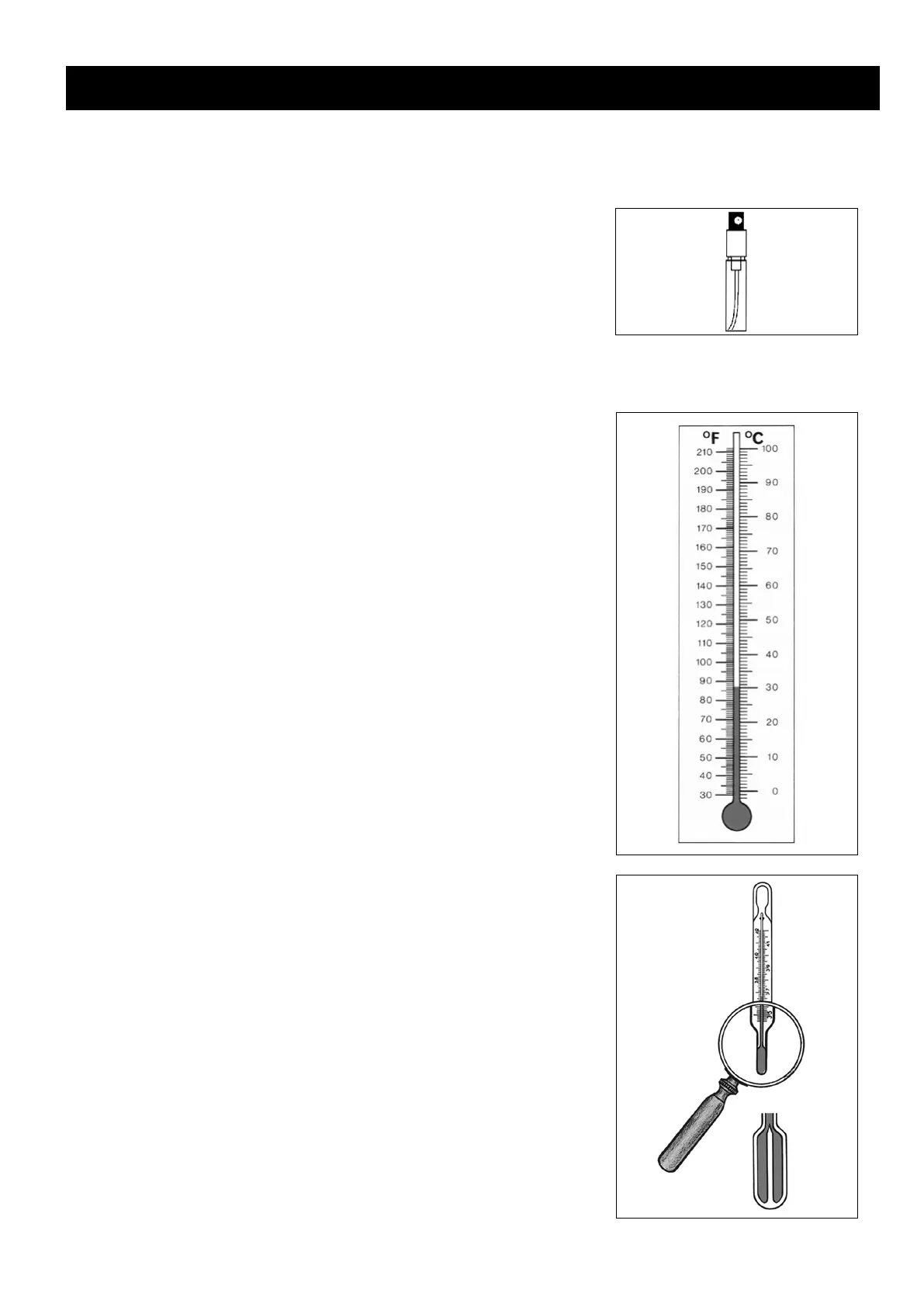
On the Celsius thermometer scale the freezing point of water is marked at
zero degrees Celsius (0 °C), and the boiling point of water at 100 degrees
Celsius (100 °C). On the Fahrenheit thermometer scale the freezing point
of water is shown as +32 °F and the boiling point of water as +212 °F. Read-
ings in degrees Fahrenheit are still used in Britain and North America.
The clinical thermometer
Whereas outdoor, room and bath thermometers always show the tempera-
ture to which they are being exposed, the clinical thermometer is a so-called
maximum thermometer. In other words, once the reading has reached its
highest level, it remains there.
This is achieved by means of a constriction between the bulb and the tube.
As the mercury expands it is able to pass through this constriction, but as it
cools it retracts, causing a break in the thread of mercury at this point. This
enables a permanent reading.
Only by shaking the clinical thermometer the liquid is forced back
through the contriction into the bulb.

 Loading...
Loading...