Heat
9
1.8 Experiment 8
Water evaporates, vaporizes too
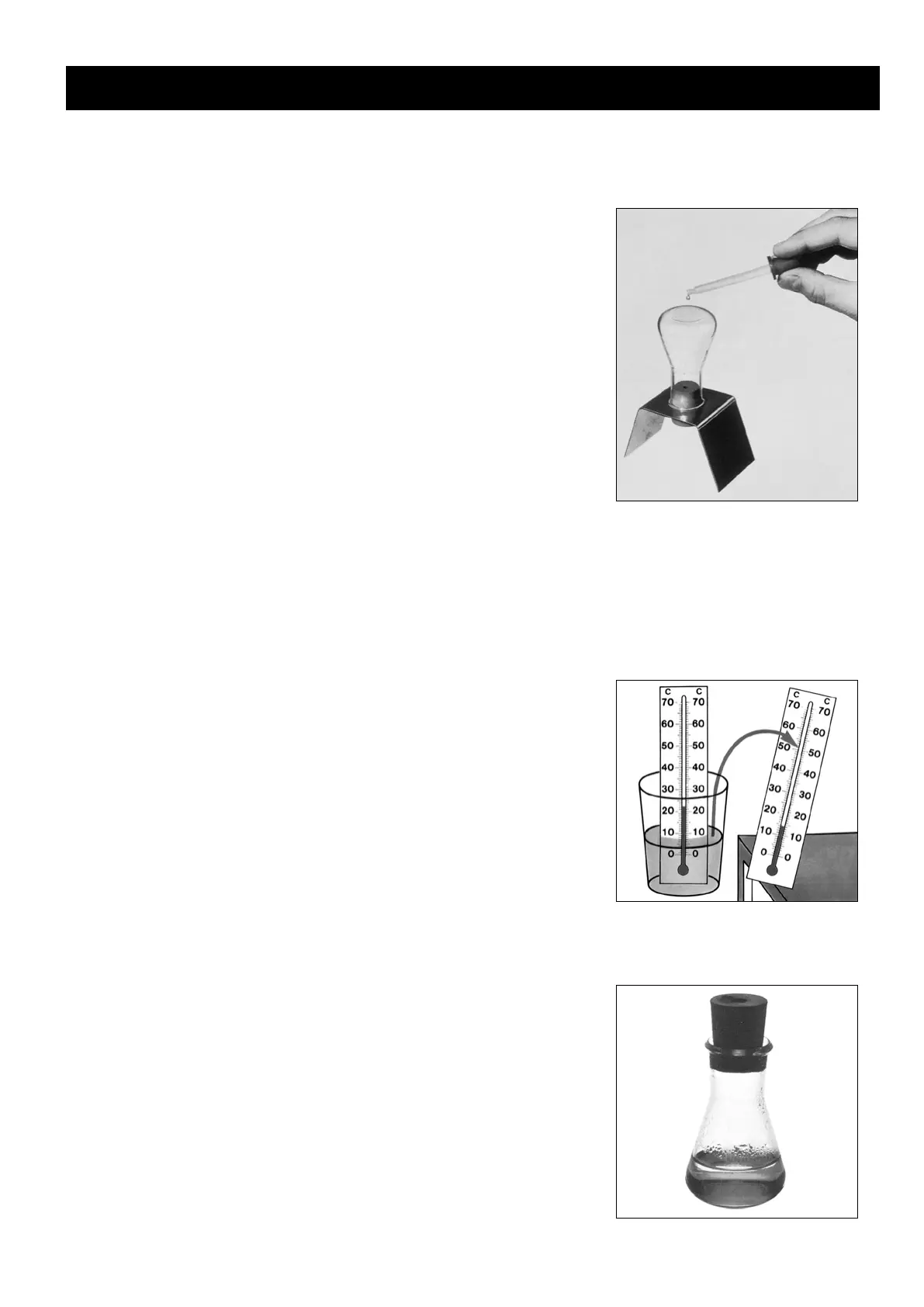
The Erlenmeyer flask is set up as in experiment 7 but now a drop of water
is placed on its base.
In this case too, the drop of water evaporates after a while even though this
happens slower than it did in experiment 7. (Different liquids evaporate
at different speeds.)
Examples of water vaporization/evaporation in every-day life:
•Wetlaundrydries
•Rainwateroncarwindows
(the drops gradually become smaller and smaller until the window is
completely dry)
•Waterinanaquariumevaporates
(water must be added from time to time)
Materials: 1 dropper (2)
1 Erlenmeyer flask (10)
1 rubber stopper (18)
1 bridge-shaped stand (19)
Additionally: some water
1.9 Experiment 9
Vaporizing liquids are cooling
With a thermometer you determine the room temperature and the tempera-
ture of methylated spirits (inflammable!) filled in a plastic beaker.
As the thermometer is pulled out of the liquid the temperature shown by
the thermometer is decreasing. (Due to the evaporating methylated spirits
on the thermometer.)
This cooling effect can also be felt by wetting a finger with methylated
spirits.
Materials: 1 thermometer, -3 °C to +103 °C (5)
1 plastic beaker (9)
Additionally: methylated spirits
1.10 Experiment 10
Can a gas be re-converted into a liquid?
Add some hot water (at least 70 °C) to the Erlenmeyer flask. Close the flask
with a rubber stopper.
The glass fogs up. The water particles which have evaporated deposit on
the glass. A gas has been converted into a liquid.
Materials: 1 Erlenmeyer flask (10)
1 rubber stopper (18)
Additionally: 1 thermos flask or glass with very hot water

 Loading...
Loading...