C3+ IFU - 0723 01140 (70048) UK Page 15 of 18
● Running water
● Strong electromagnetic forces
5.3 Warranty
The C3+ has a warranty of 2 years from the date of purchase.
5.4 Disposal
The C3+ should be decommissioned when it reaches the end of its service life. The C3+ should be
disposed of in accordance with the EU WEEE directive for electronic waste.
1
6. Technical and Regulatory information
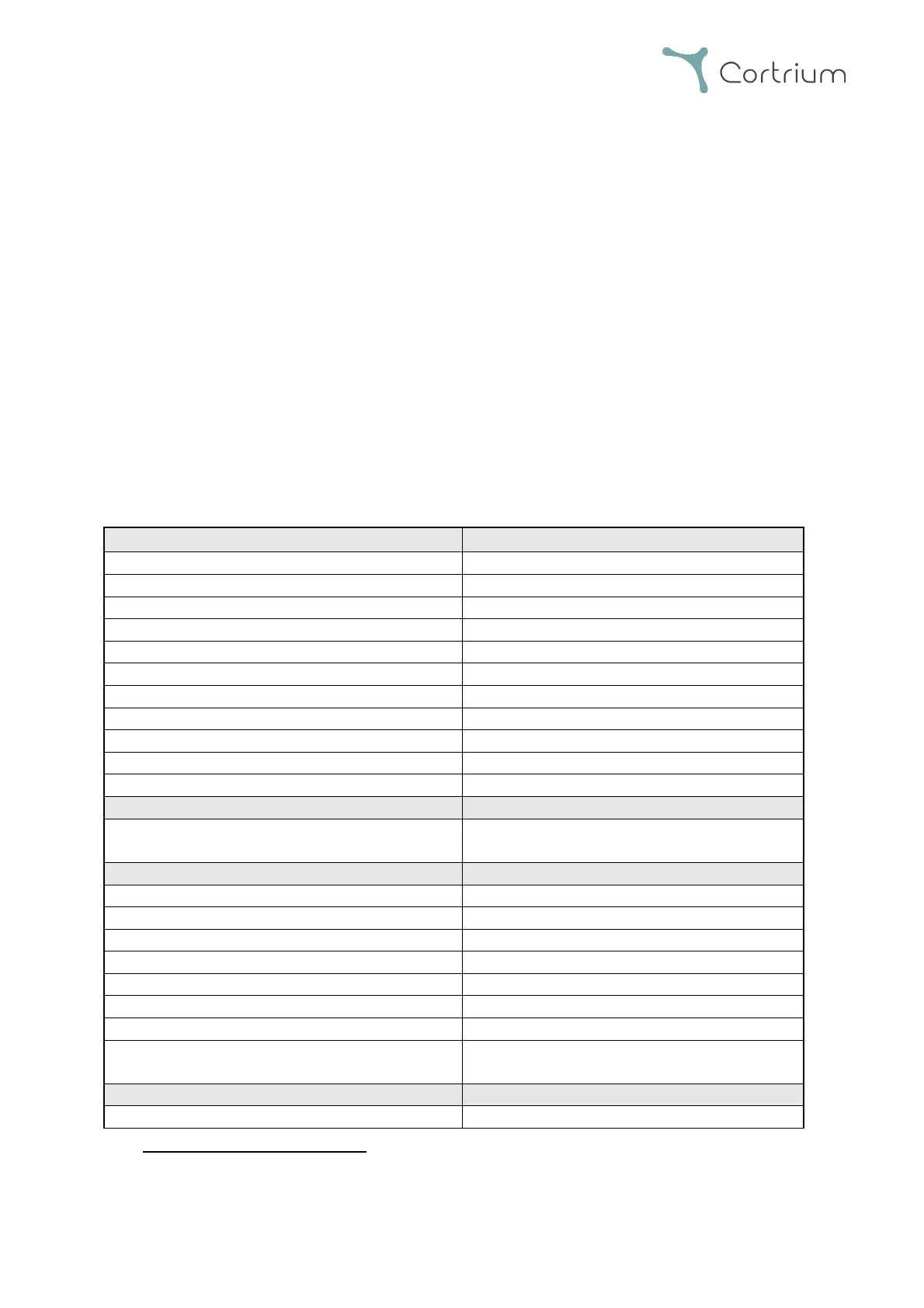
6.1 Technical specification
Lithium Polymer, 3.7V, 520 mAh
Design verification IEC 60601-2-47
IEC 60601-1 Basic Safety & Essential
Performance IEC 60601-2-47
Body fluids contacted by device
Type of contact to intact skin
Up to 7days continued contact
1
DIRECTIVE 2012/19/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 4 July 2012 on waste
electrical and electronic equipment (WEEE).

 Loading...
Loading...