4
BEFORE USE
Please read these Operating Instructions carefully as they
explain all the most important details and procedures. Please
pay special attention to the safety precautions.
Always keep these Instructions close at hand.
These Instructions are only applicable to the equipment
they were delivered with.
To prevent injury to persons or damage to property, please
observe the corresponding directives and symbols.
This instruction follows the recommendations of RKI - Robert
Koch Institut, Germany.
Translations in this manual are based on the English version.
Thus the English text will be referred to in case of discrepancy.
Caution / Danger
Risk of property damage or injury
Refer to instruction manual
Please note
Useful additional information and hints
Sterilizable at up to 135°C in the autoclave
Prohibited Suitable for thermal disinfection
Wear personal protective equipment
Required time
Abbreviations used: min (minute) ; s (second)
SYMBOLS
DEVICES AND HANDPIECE CORDS
The following instructions have been validated for all reusable products
1
of EMS dental devices. It remains the responsibility of
the processor to ensure that the processing, as actually performed using the equipment, materials and personnel in the processing
facility, achieves the desired result. This requires validation and/or validation and routine monitoring of the process. Likewise any
deviation by the processor from the instructions provided should be properly evaluated for effectiveness and potential adverse effects.
INTRODUCTION
EMS recommends compliance with cleaning, disinfection,
packaging for sterilization and sterilization procedures according
to ISO 17664.
Always report adverse events related to device reprocessing
directly to EMS.
Products must be cleaned, disinfected and, if applicable,
the authorized number of sterilization cycles. To replace, refer
to the “Service life” section of these Instructions.
Concentrations and contact times specified by the
manufacturer of the cleaning and disinfection agent must be
observed.
Remember that sterilization cannot be achieved unless
If parts of these instructions are not clear or seem to be
inadequate, do not hesitate to contact/inform EMS.
Users shall also observe any legal requirements applicable in their country as well as the hygiene regulations in effect
at the hospital or clinic.
This applies especially with regard to additional requirements for the inactivation of prions.
SERVICE LIFE
The products have been designed for a large number of
sterilization cycles. The materials used in their manufacture
were selected accordingly. However, with every renewed
preparation for use, thermal and chemical stresses will result
in ageing of the products.
Always replace products that present signs of wear or
early degradation, regardless of the number of sterilization
cycles left unused.
DO NOT expose the products to temperature exceeding
138°C.
Lifetime : see product's Instructions for Use.
1
Hereinafter referred to as "products".
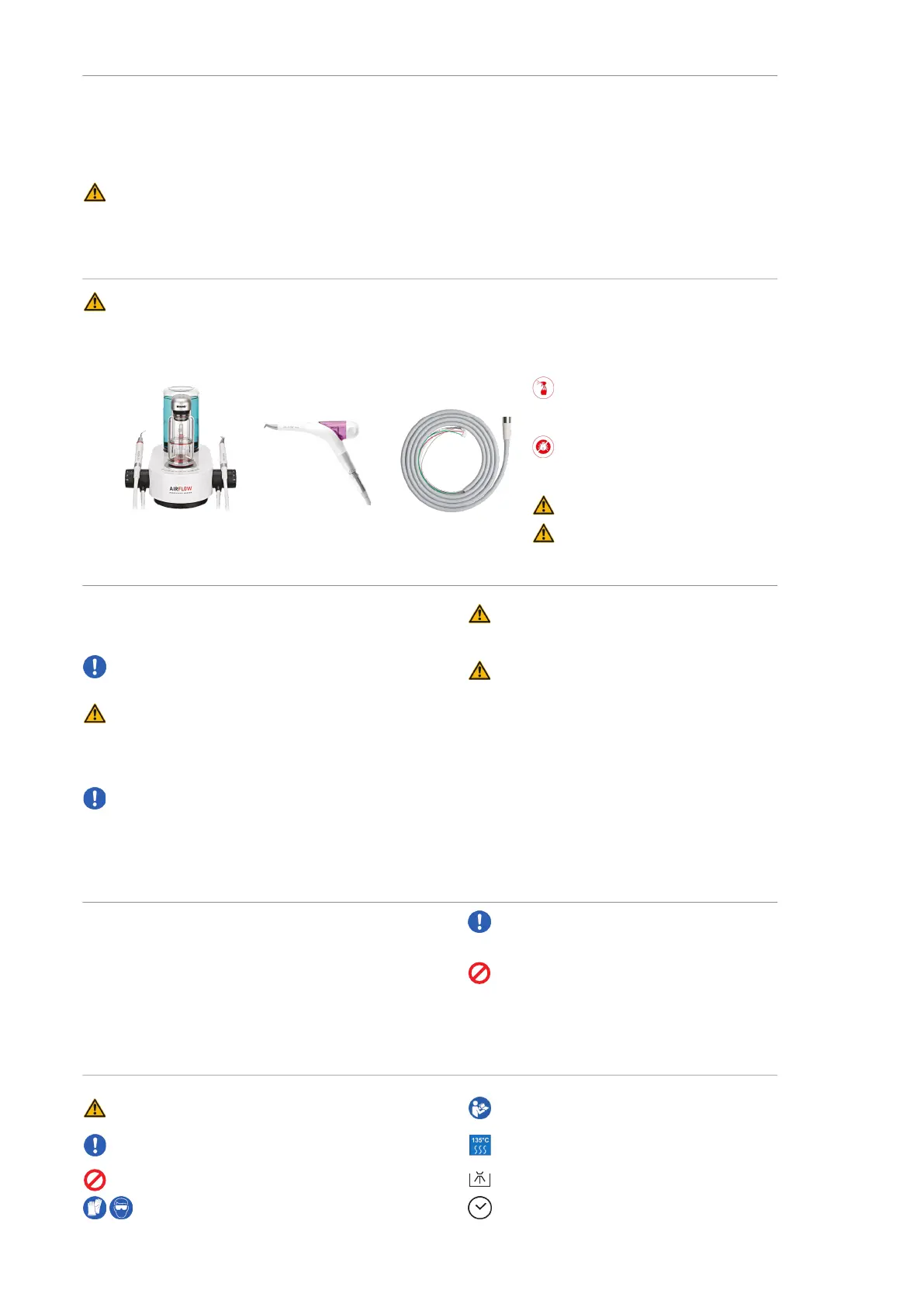
AIRFLOW
®
and PIEZON
®
devices Handy PIEZON
®
Kit on your unit
CLEAN
Wipe the surface with a wet cloth until all visible
contamination is removed.
DISINFECT
Wipe the surface with 70% isopropyl alcohol or a
Follow carefully the instructions provided by the
disinfection solution manufacturer.
Do not use CaviWipes™. It damages EMS products.
ENGLISH

 Loading...
Loading...