Do you have a question about the Fresenius Medical Care multiFiltrate and is the answer not in the manual?
Explains document identification, text styles, and illustration usage for effective manual navigation.
Provides a brief description of the device, its intended purpose, and specifications for use.
Details various CRRT treatment modes and methods for anticoagulation of the extracorporeal blood circuit.
Covers contraindications, risks, target group, operator duties, and general safety warnings.
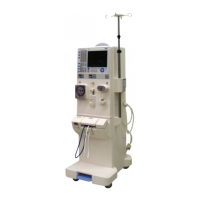
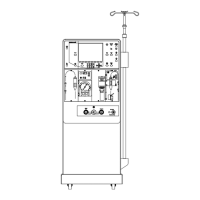
Provides visual representations of the device.
Shows a labeled diagram of the device's front components.
Shows a labeled diagram of the device's rear components.
Details major components like Monitor, Heparin Pump, Extracorporeal Module, and Ci-Ca Module.
Covers general principles for device application, including network connection and safety precautions.
Explains the device's operational concept, menu navigation, and parameter entry.
Details steps for setting UF goal, alarm limits, preparing the device, and selecting modes.
Guides through CRRT setup, preparation, treatment execution, and disconnection procedures.
Explains how to acknowledge messages and the hazards of ignoring alarm causes.
Describes the 'Old' and 'New' alarm schemes and their assignment principles.
Details how the system responds to alarms, including overriding and suppressing audible alarms.
Explains message boxes, handling alarm limits, and key alarm ratios.
Details the procedure for surface cleaning and disinfection after each treatment.
Lists disinfectants and cleaning agents tested and recommended for device use.
Provides an overview of the device's main functions like blood circuit, balancing, and alarms.
Describes CRRT, Paediatric CRRT, MPS, SCUF, and Haemoperfusion treatments.
Explains systemic anticoagulation and regional citrate anticoagulation methods.
Lists multiFiltrate kits, haemofilters, solutions, syringes, and other disposables.
Lists available accessories like plasma poles and additional equipment such as cables.
Details environmental and power supply network requirements for device installation.
Covers battery charging and important information for initial device start-up.
Addresses electrical safety, installation, EMC, and power cable requirements.
Provides guidance on safely moving, locking, and transporting the device.
Details storage conditions, battery charging, and maintenance when the device is not in use.
Outlines information for responsible organizations and recycling facilities regarding device disposal.
Provides important information on Technical Safety Checks (TSC) and Maintenance Procedures (MA) timing.
Specifies the required timing for the first and subsequent Technical Safety Checks.
Recommends maintenance procedures to ensure trouble-free operation within specified intervals.
Defines the qualification requirements for personnel performing safety checks and maintenance.
Details device dimensions, weight, load capacity, and identification labels.
Covers electrical safety, supply details, and electromagnetic compatibility guidelines.
Includes operating/storage conditions, connection options, and safety system specifications.
Lists the various plastics, metals, electrical components, and lacquers used in the device.
Provides definitions for various terms related to haemodialysis and device operation.
Lists common abbreviations used in the manual and explains device symbols.
States that EC certificates can be obtained from the local service support organization.
Details Ci-Ca module operation, anticoagulation, completion, and alarm management.
Covers mDL requirements and procedures for entering patient and case IDs.
Addresses network operator responsibilities for device protection and data security.
Provides information on free software licenses and usage within the haemodialysis system.
| Brand | Fresenius Medical Care |
|---|---|
| Model | multiFiltrate |
| Category | Medical Equipment |
| Language | English |