Electrical safety tests
Ultrasound System – Common Service Information 4-27
Direction 5444964-100 English Rev. 5
Data sheet for enclosure/chassis leakage current
Table 4-10 shows a typical format for recording the enclosure/
chassis leakage current. Measurements should be recorded
from multiple locations for each set of test conditions. The actual
location of the test probe may vary by Ultrasound system.
Record all data in the Electrical safety tests log.
NOTE: Not all test procedures are applicable to all areas of the world.
Reversed Polarity testing content satisfies regions following IEC
62353:2007 and IEC 60601-1:2005.
NOTE: Values in italics font are given as examples only.
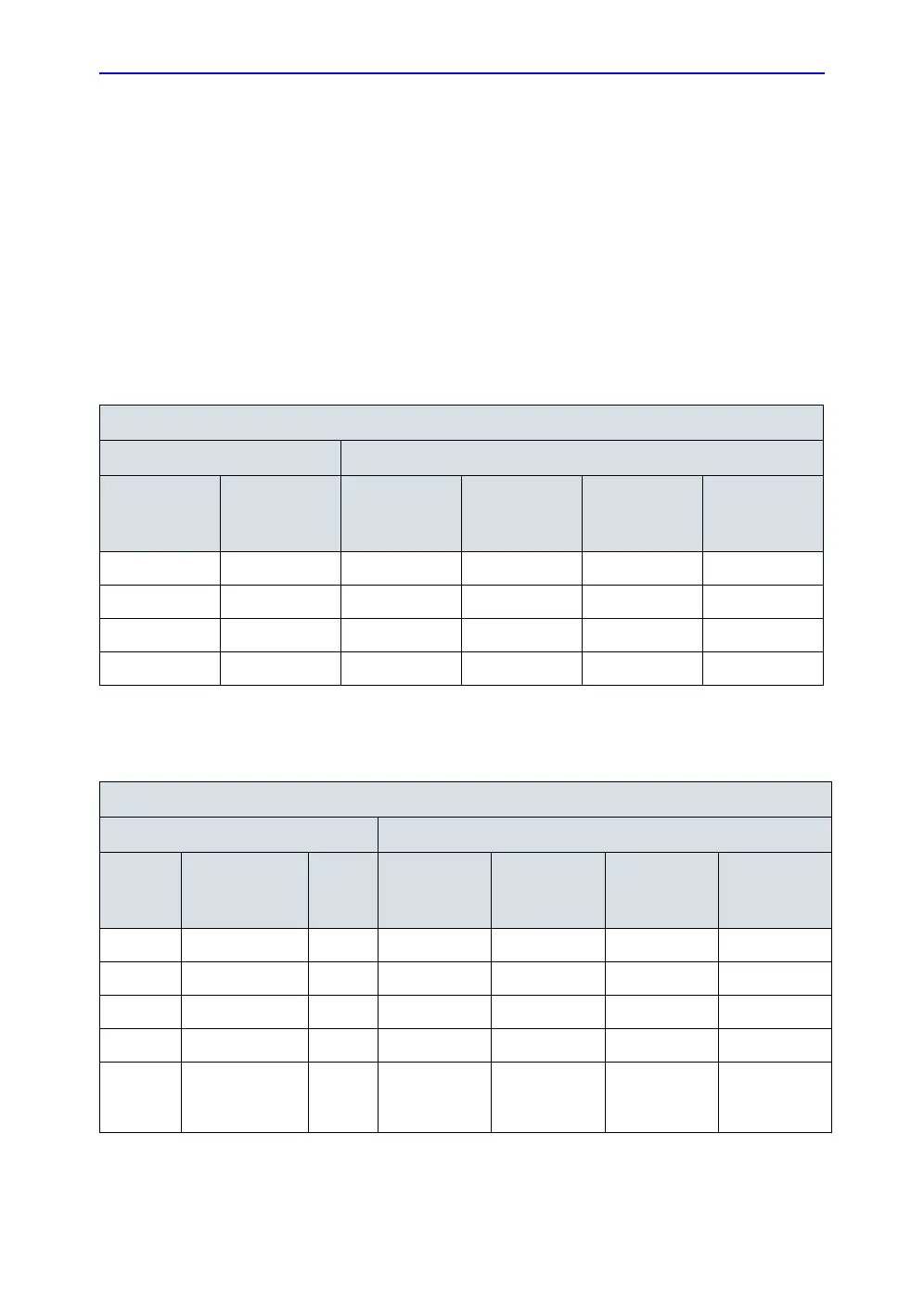
Table 4-10: Typical data format for recording enclosure/chassis leakage
Unit under test____________________________________ Date of test:_____________
Test Conditions Measurement/Test Point Location
System
Power
Grounding/
PE
Potential
equilibrium
connectorl
Lower
Frame
Probe
Connector
Main
Handle
off closed
off open
on closed
on open
Table 4-11: Typical data format for recording enclosure/chassis leakage
Unit under test____________________________________ Date of test:_____________
Test Conditions Measurement/Test Point Location
System
Power
Grounding/
PE
Limit
µA
Potential
equilibrium
connector
Monitor
housing
Probe
connector
off closed 100
off open 500
on closed 100
on open 500
off closed
(reversed
polarity)
100

 Loading...
Loading...