STORAGE OF EQUIPMENT
If you do not use the device for a short period of time, store it in the supplied case
after turning it off and cleaning it if necessary. Try not to twist and fold the air tube and
cuff too tightly.
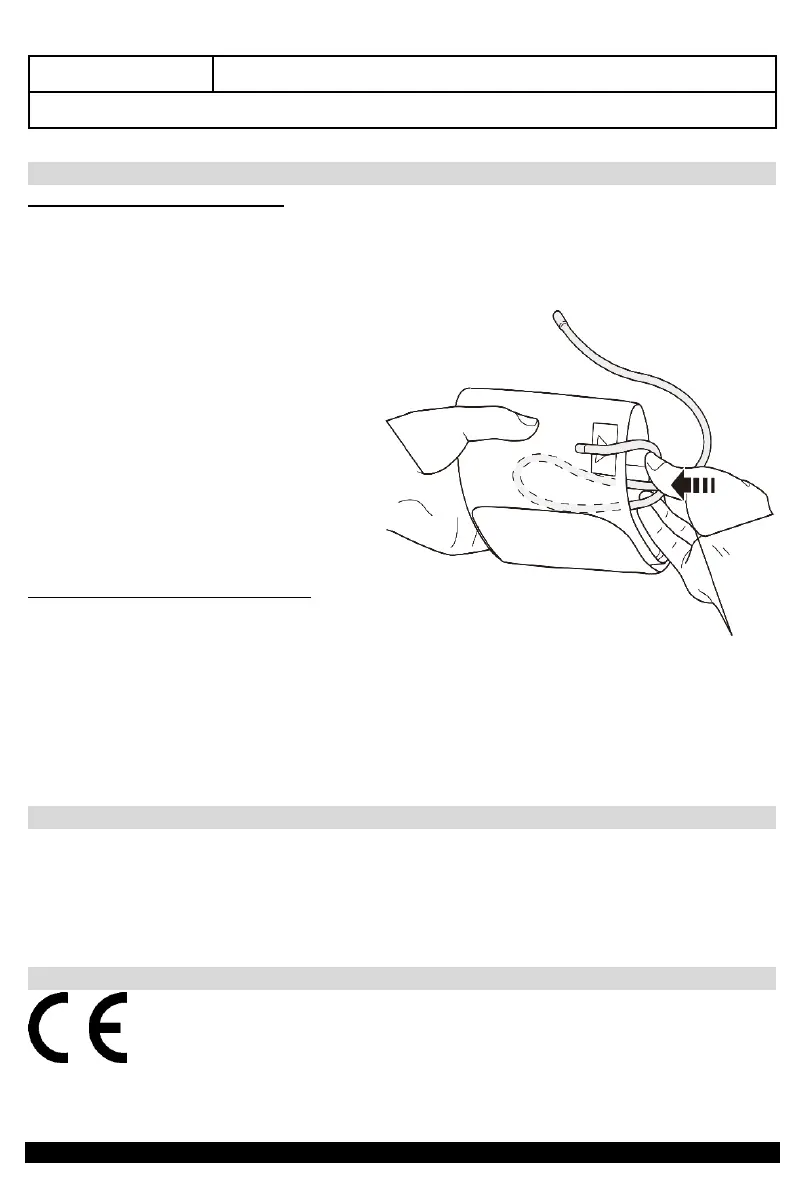
If you are planning not to use the
device for a long time clean it,
disconnect the air tube and put it in a
slightly rolled-up cuff (as shown in the
illustration).Removie the battery pack,
store it in the supplied case in a dry
room away from heat and moisture
sources. It is best to place the device
in its original packaging, away from
children and pets.
TRANSPORT OF THE DEVICE
When handling, only carry the unit
after it has been switched off, holding
it securely by the housing and the cuff.
It is recommended to transport the device only when the following conditions have
been met: Clean the device, remove the battery pack and disconnect the air tube from
the device. Put the device into the case and preferably into the original packaging. Do
not expose the unit to excessive vibration, shock, heat, humidity or extreme
temperatures during transportation
The product is covered by a 2 year warranty from the date of sale of the product.
Batteries are not covered by the warranty - it is a consumable material. If you need
warranty repair, please contact the manufacturer's hotline. The product returned for
repair should be complete and preferably in its original packaging.
This device meets the requirements of the European Commission
Directive 93/42/EEC on Medical Devices. This blood pressure monitor is
designed in accordance with European Standard EN1060, Non-invasive
Sphygmomanometers, Part 1, General Requirements, and Part 3.

 Loading...
Loading...